Human mesenchymal stem cell preservation transportation liquid and application thereof
A technology of mesenchymal stem cells and transport fluid, applied in the field of human mesenchymal stem cell preservation and transport fluid, can solve the problems of cell mass embolism of blood vessels, risk of patient infusion, death and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
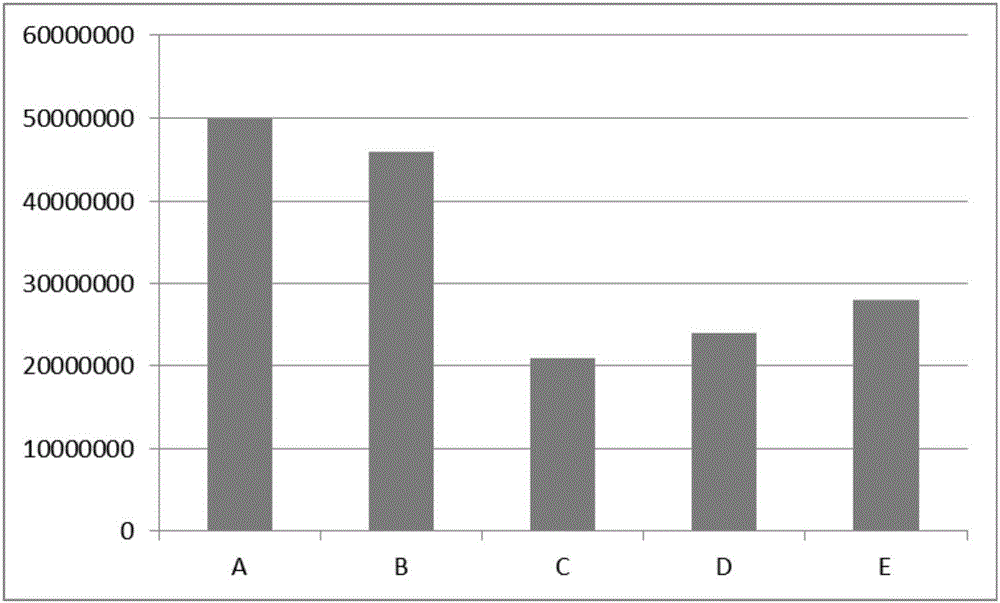
[0024] The effect of serum concentration of human AB on cell adhesion
[0025] The umbilical cord mesenchymal stem cells were prepared by the tissue block adherence method, and after three passages, the cells were collected by centrifugation, and the supernatant was removed. Prepare 5 groups of stem cell storage and transportation solutions, all of which contain 5.26mg / ml sodium chloride, 5.02mg / ml sodium gluconate, 3.68mg / ml sodium acetate, 2.5mg / ml ginsenoside Rb1, 10ng / ml transferrin , 10ng / ml reduced glutathione, 0.1mmol / l glutamine, wherein 2v / v% human AB serum in group A; group B: 5v / v% human AB serum; group C: 20v / v% human AB serum; D group: 50v / v% human AB serum; E group: 20v / v% human serum albumin. Except for the difference in serum concentration, the other components of each group were the same. Dilute the above-prepared umbilical cord mesenchymal stem cells into 5х10 7 / ml. 1ml of each group was stored statically in a 4°C incubator for 24 hours, then transferred...
Embodiment 2
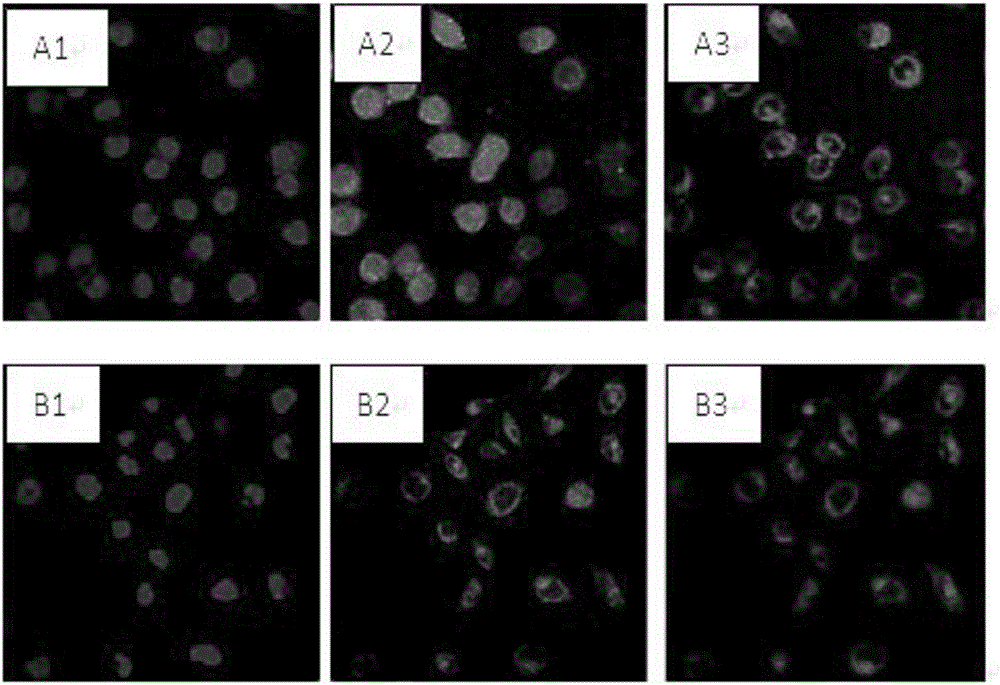
[0027] Effects of transferrin and reduced glutathione on cellular ROS levels
[0028] The umbilical cord mesenchymal stem cells were prepared by the tissue block adherence method, and after three passages, the cells were collected by centrifugation, and the supernatant was removed. Prepare 2 groups of stem cell storage and transportation solutions, both of which contain 5.26mg / ml sodium chloride, 5.02mg / ml sodium gluconate, 3.68mg / ml sodium acetate, 2v / v% human AB serum, 2.5mg / ml ginsenoside Rb1, 0.1mmol / l glutamine, where A: without transferrin and reduced glutathione; B: with 10ng / ml transferrin and 10ng / ml reduced glutathione. Except for transferrin and reduced glutathione, the other components of the two groups were the same. Dilute the above-prepared umbilical cord mesenchymal stem cells into 5х10 7 / ml. 1ml of each group was statically stored in a 4°C incubator for 24 hours, centrifuged to remove the preservation solution, and the cells were washed three times with PBS...
Embodiment 3
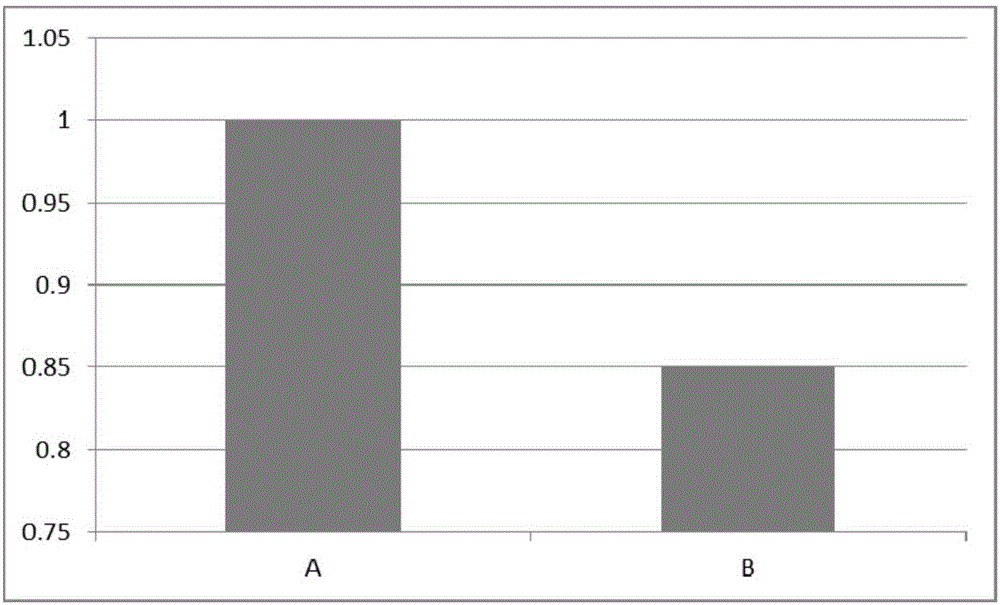
[0030] Ginsenoside Rb1 can effectively maintain cell viability and metabolism
[0031] Conventional MTT method and PI staining method can only measure apoptotic and necrotic cells and non-apoptotic cells. In fact, many cells are irreversible when the environmental conditions are not suitable and their own regulation mechanism is not enough to regulate. It will gradually move from the normal state to apoptosis, and there is still a process from the beginning of apoptosis to necrosis, generally ranging from a few hours to a day or two depending on the treatment conditions. During this process, the cell membrane is still intact, PI cannot be stained, and the cell viability will change, but it can still maintain a certain metabolic capacity. However, at this time, the synthesis ability and content of ATP in the cell will change significantly. Therefore, ATP The content change of can best reflect the change of cell metabolism state.
[0032] The umbilical cord mesenchymal stem cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com