Fingerprint construction method and HPLC (High Performance Liquid Chromatography) fingerprint
A technology of fingerprint spectrum and construction method, applied in the field of Panax notoginseng drug analysis, can solve the problem of not being able to distinguish the medicinal parts of Panax notoginseng well, and achieve the effect of good stability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
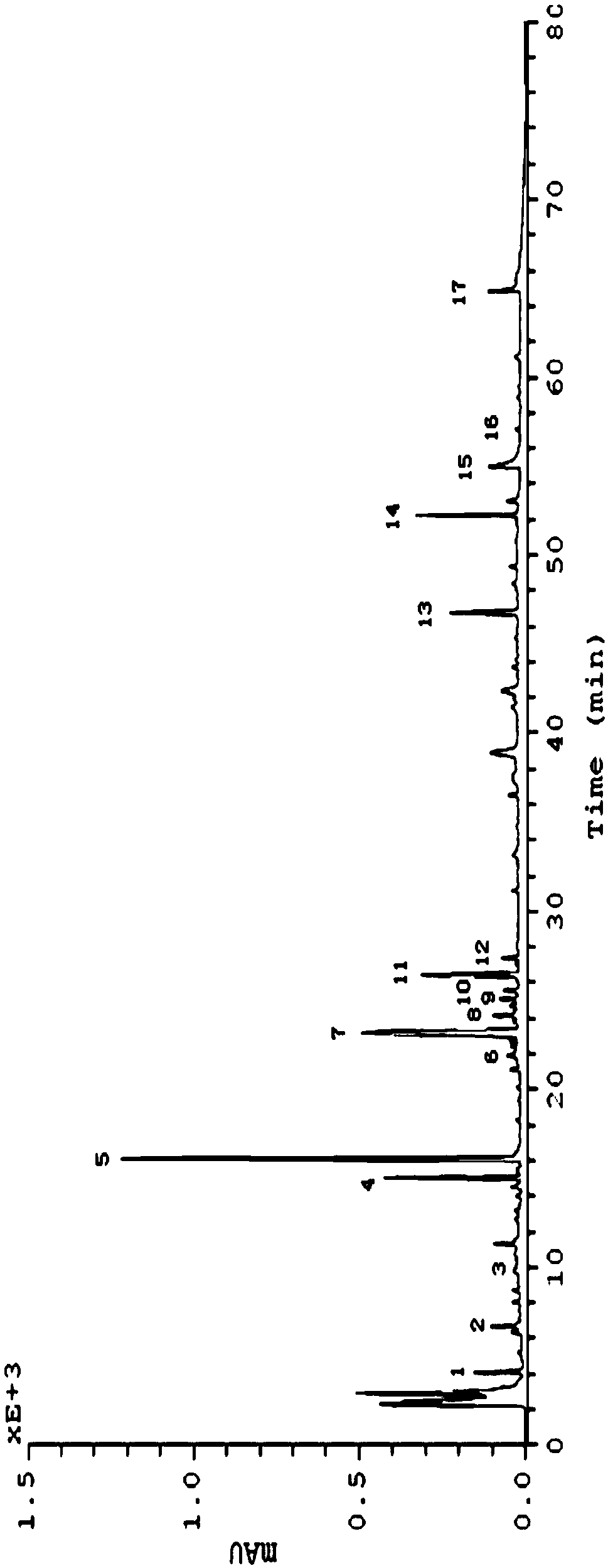
[0032] 1. Chromatographic conditions
[0033] Column: Kromasil C 18 Chromatographic column (250×4.6mm, 5μm, ); mobile phase: acetonitrile (A): water (B) linear gradient elution (v / v), the ratio is shown in Table 1; flow rate: 1mL min -1 ; Column temperature: 30° C.; Detection wavelength: 203 nm. Injection volume: 20 μL.
[0034] Table 1: Acetonitrile (A): water (B) mobile phase gradient table (v / v)
[0035] time
0~5min
5~20min
20~45min
45~50min
50~60min
60~70min
Acetonitrile (%)
5→20
20→36
36→80
80→100
100
100→5
water(%)
95→80
80→64
64→20
20→0
0
0→95
[0036] 2. Preparation of reference substance and test solution
[0037] Preparation of reference solution: Accurately weigh appropriate amount of notoginseng saponin R 1 , Ginsenoside Rg 1 , Re, Rb 1 , Rb 2 , Rb 3 and Rf reference substance, dissolved in methanol to make a concentration of about 50μg·mL -1 mixed reference solution. ...
Embodiment 2
[0046] 1. Precision experiment
[0047] Accurately draw the notoginseng need testing solution in the embodiment one, measure by the chromatographic condition in the embodiment one, respectively continuous sampling 5 times, with notoginseng saponin R 1 The relative retention time and peak area ratio of the characteristic peaks in the obtained spectrum were analyzed for the reference peak (S peak), and the RSD was calculated. Among them, the RSD of the retention time of the S peak was 0.14%, the RSD of the peak area was 0.46%, the RSD of the relative retention time of other characteristic peaks was 0.013%-0.21%, and the RSD of the relative peak area was 0.29%-3.44%.
[0048] 2. Stability experiment
[0049] Accurately draw the notoginseng need testing solution in embodiment one, place respectively after 1,2,3,5,7 days, measure fingerprint by the chromatographic conditions in embodiment one, with notoginseng saponin R 1 The relative retention time and peak area ratio of the cha...
Embodiment 3
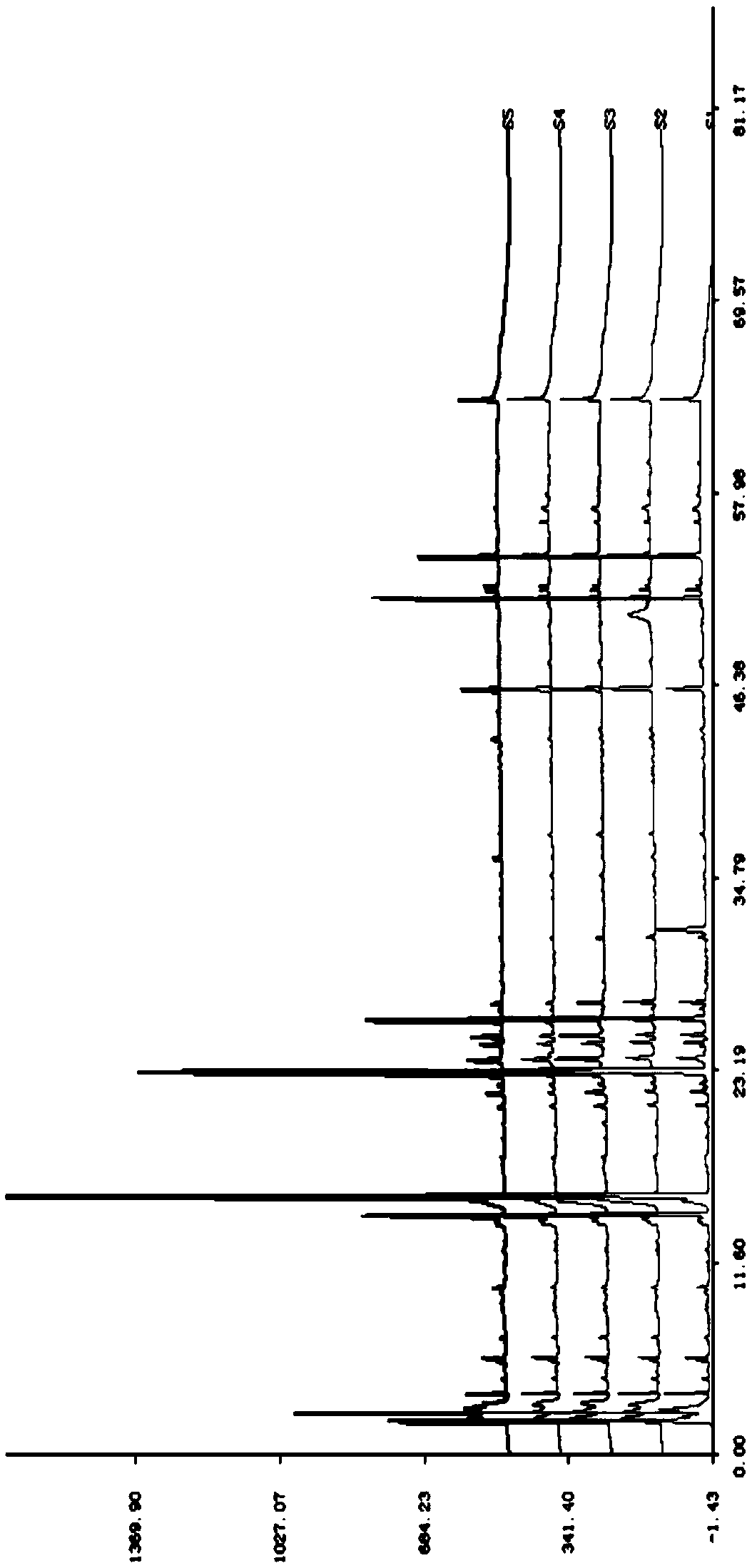
[0054] Accurately weigh 20, 40, 60, 80, 100 heads of Panax notoginseng main root, tendon (branch root), scissors (rhizome), leaf and flower coarse powder 2g respectively, a kind of need testing solution preparation method according to embodiment Prepared, measured by chromatographic conditions in Example 1, the results are shown in image 3 and 4 . Take notoginseng saponin R1 as the reference peak (S peak) to analyze the relative retention time and peak area ratio of the characteristic peaks in the spectra obtained. The results are shown in Table 3 and Table 4.
[0055] Table 3. The relative retention time of the main peaks in the HPLC fingerprints of Panax notoginseng with different specifications and different medicinal parts
[0056]
[0057] Table 4. The relative peak area ratio of the main peaks in the HPLC fingerprints of Panax notoginseng with different specifications and different medicinal parts
[0058]
[0059]
[0060] As can be seen from Tables 3 and 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com