Electrochemical analysis device adopting nano boron-doped diamond film electrode and its uses
An electrochemical analysis, boron-doped diamond technology, applied in the field of electrochemical analysis device, high-sensitivity detection of heavy metal ions, can solve problems such as insufficient detection sensitivity of metal ions, achieve good anti-oxidative interference ability, simple equipment, and sample dosage less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
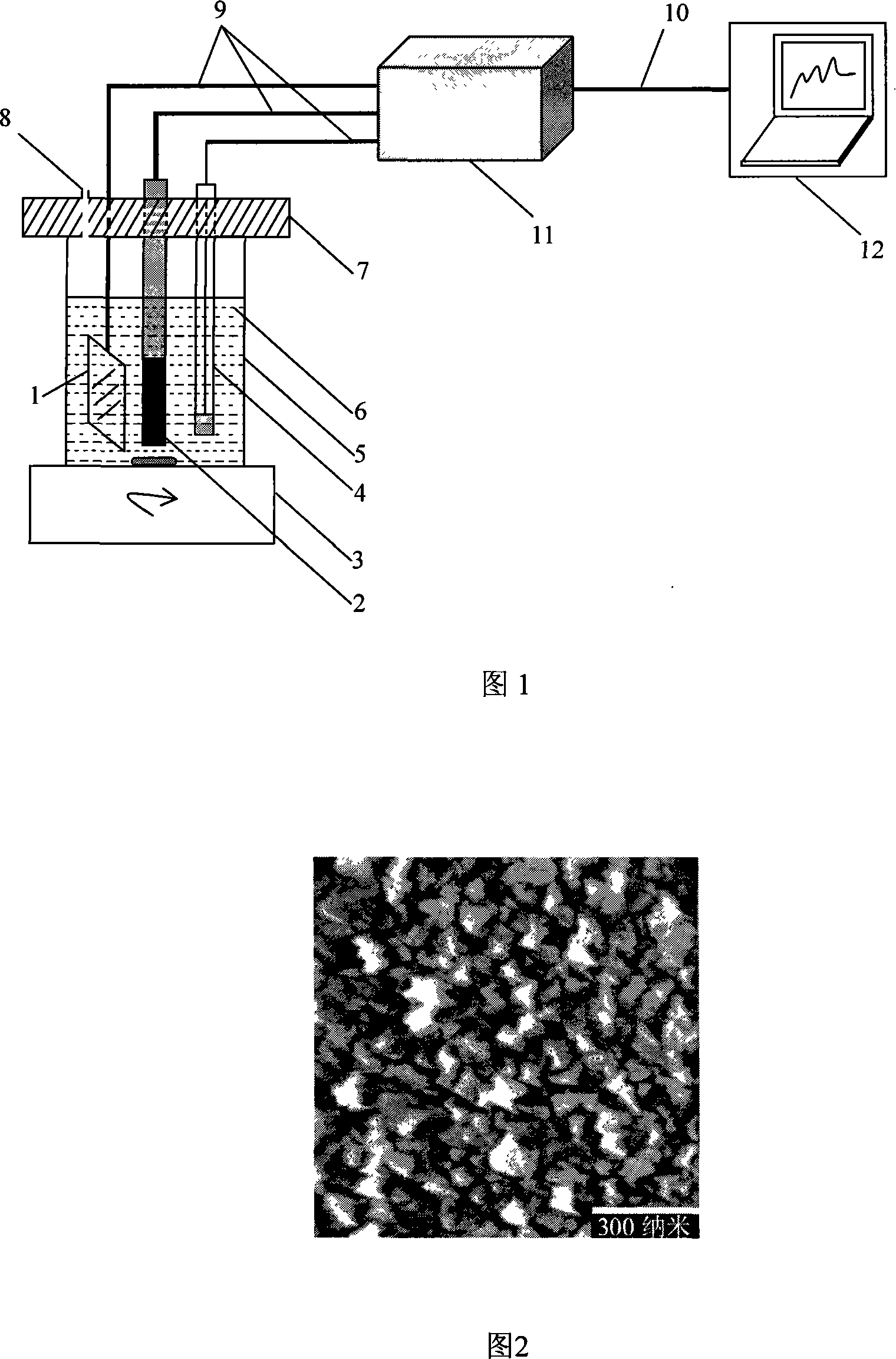
[0019]The nanometer boron-doped diamond film electrode that the present invention adopts refers to people such as Greg M.Swain in literature (Direct Electrochemistry of Cytochrome c at Nanocrystalline Boron-Doped Diamond, Shannon Haymond, Gerald T.Babcock, and Greg M.Swain, J .AM.CHEM.SOC.2002, 124, 10634-10635) reported in the microwave-assisted chemical vapor deposition method (adjusted in specific parameters) prepared. Specifically as solid B 2 o 3 As a boron doping source, the silicon substrate is placed in the reaction chamber after conventional treatment, and after being evacuated, the CH 4 (2%) and Ar (98%) mixed gas is input to the vicinity of the tungsten wire installed in the tube, and the tungsten wire is heated to above 2000°C with a DC stabilized voltage power supply, the substrate temperature is about 500-900°C, and the indoor gas pressure The temperature is 103-105Pa, and the growth time is 1h; then the sample is transferred to the microwave CVD reaction chamb...
Embodiment 2
[0022] Adopt the electrochemical analysis device of embodiment 1, put into the H of 1.0mol / L in electrolytic cell 5 earlier 2 SO 4 The solution is used as the electrolyte 6, and the working electrode 2 (nano-BDD electrode) is placed in the H 2 SO 4 The solution was electrolyzed at a potential of 2.8V (Vs.SCE) for 15s, and then, the working electrode 2 was 2 SO 4 The cyclic voltammetry scan was carried out in the solution, the potential range was -2.0 to +3.0V, and the scan rate was 50mV / s until a stable cyclic voltammogram was obtained.
[0023] Next, prepare 0.01mol / L Cd(NO 3 ) 2 , 0.01mol / LPb(NO 3 ) 2 , 0.01mol / LCu(NO 3 ) 2 , 0.01mol / L Ag(NO 3 ), 0.20mol / L HAC-NaAC (pH=4.6) solution. And according to the needs of the experiment, use 0.20mol / L HAC-NaAC (pH=4.6) buffer solution to dilute the metal nitrate solution prepared above step by step to obtain a series of metal ion salt solutions with different concentrations. And replace the H in the electrolytic cell 5 in...
Embodiment 3
[0029] The first step is the same as example 2, at first adopting constant potential electrolysis, the nano-boron-doped diamond film electrode is in the H of 1.0mol / L 2 SO 4 The solution was electrolyzed at a potential of 2.8V (Vs.SCE) for 15s, and then the electrode was placed in H 2 SO 4 The cyclic voltammetry scan was carried out in the solution, the potential range was -2.0 to +3.0V, and the scan rate was 50mV / s until a stable cyclic voltammogram was obtained.
[0030] Then, use double distilled water to prepare 0.01mol / L SnCl 2 , 0.01mol / L Cu(NO 3 ) 2 , 0.01mol / LAg(NO 3 ), 0.03mol / L HNO 3 solution. And according to the experimental needs, use 0.03mol / L HNO 3 The above prepared 0.01mol / L SnCl 2 , 0.01mol / L Cu(NO 3 ) 2 , Ag(NO 3 ) solution was diluted step by step to obtain a series of metal ion salt solutions with different concentrations. And replace the H in the electrolytic cell 5 in sequence 2 SO 4 solution as electrolyte 6.
[0031] Using anodic stri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com