Antineoplastic drug tetrahydronaphthalene amide compound and pharmaceutically acceptable salt thereof, preparation method and application thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as life-threatening and serious conditions of patients, and achieve the effect of wide therapeutic window, broad anti-cancer spectrum, excellent anti-tumor activity and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
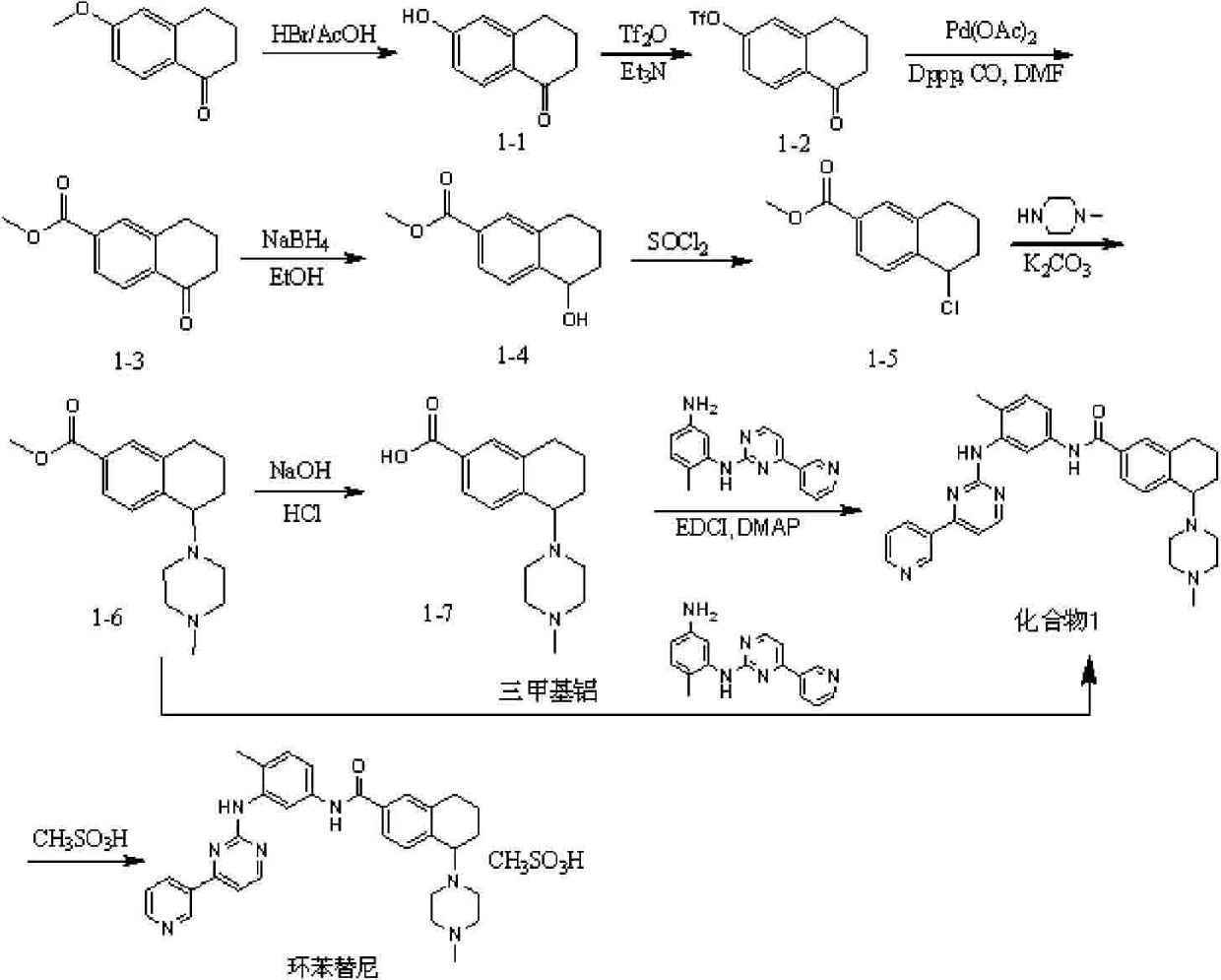
[0052] Example 1 Preparation of compound 1 and pharmaceutically acceptable salt thereof
[0053] (one) N-{4-methyl-3-[(4-pyridin-3-yl)pyrimidine-2-amino]phenyl}-5-(4-methylpiperazin-1-yl)-5,6,7, The preparation of 8-tetrahydronaphthalene-2-amide (compound 1), the structural formula of compound 1 is as follows:
[0054]
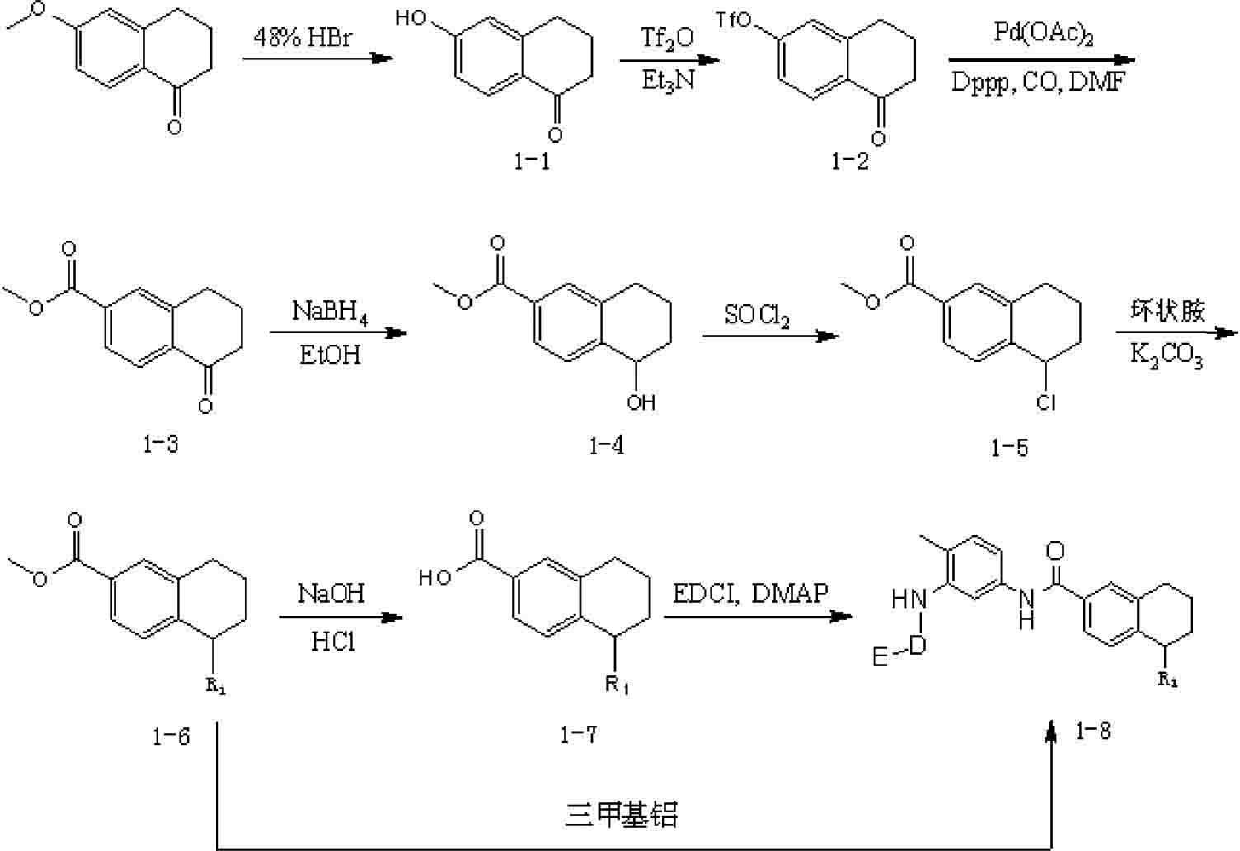
[0055] Step A: Synthesis of 6-Hydroxy-1-tetralone
[0056]
[0057] 6-Methoxyl-1-tetralone (17.6 g, 100 mmol) was reacted in 100 ml of 48% hydrogen bromide solution at 100°C for 24 hours and then cooled to room temperature. Solid precipitated, filtered out the solid and rinsed with 5% aqueous sodium bicarbonate solution, then washed with deionized water until neutral, dried, and the crude product was purified with silica gel column, and eluted with ethyl acetate:n-hexane=1:5 to obtain the product 13.9 grams, yield 86.0%. MS(M+1)=163.14.
[0058] Step B: Synthesis of 5-carbonyl-5,6,7,8-tetrahydronaphthalene-2-trifluoromethanesulfonate
[0059...
Embodiment 2
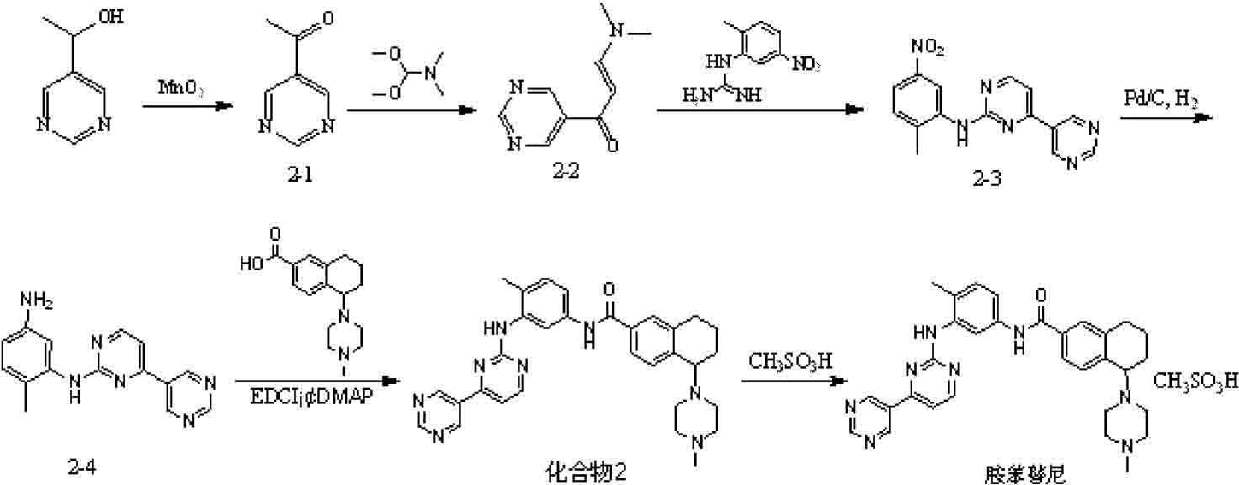
[0087] Example 2 4-{6-[({4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl}amino)carbonyl]-1,2,3,4 -Preparation of tert-butyl tetrahydronaphthalene-1-yl}piperazine-1-carboxylate
[0088] Its structural formula is as follows:
[0089]
[0090] Step A: Synthesis of 4-[6-(methoxycarbonyl)-1,2,3,4-tetrahydronaphthalen-1-yl]piperazin-1-yl-carboxylic acid tert-butyl ester
[0091]
[0092] Methyl 5-chloro-5,6,7,8-tetrahydronaphthalene-2-carboxylate (22.4 g, 100 mmol), potassium carbonate (27.8 g, 200 mmol), piperazine-1-carboxylic acid Tert-butyl ester (20.5 g, 110 mmol) was added to 300 ml of DMF, stirred at 40°C for 5 hours, the insoluble matter was filtered, and the filtrate was added to 800 ml of ethyl acetate, washed with brine (3×800 ml), The organic phase was dried by adding anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. After silica gel column chromatography, the product was eluted under the condition of ethyl...
Embodiment 3
[0101] Example 3 N-{4-methyl-3-[(4-pyridin-3-yl)pyrimidine-2-amino]phenyl}-5-(piperazin-1-yl)-5,6,7,8 -preparation of tetrahydronaphthalene-2-amide
[0102] Its structural formula is as follows:
[0103]
[0104] 4-{6-[({4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl}amino)carbonyl]-1,2,3,4-tetra Hydronaphthalen-1-yl}piperazine-1-carboxylic acid tert-butyl ester (1.86 g, 3 mmol) was dissolved in 10 ml of a mixed solution of trifluoroacetic acid:dichloromethane=1:4. After stirring at room temperature for half an hour, 20 ml of aqueous sodium carbonate solution was added to adjust the pH to weakly alkaline, the organic phase was dried by adding anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 1.48 g of the product, with a yield of 95.1%.
[0105] MS(M+1)=520.22. 1 H-NMR (DMSO- d 6 ppm): δ 10.03 (s, 1H); 9.22 (s, 1H); 8.96(s,1H); 8.64(d, J=4.8 Hz, 1H); 8.52(d, J=5.2 Hz, 1H); 8.36 (d, J=8.0 Hz, 1H); 8.02(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com