Therapeutic agents for adiposity or fatty liver
A technology for the treatment of drugs and fatty liver, applied in drug combination, pharmaceutical formula, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of icariin (see Ye Haiyong et al., Zhejiang University Journal (Medical Science), 2005, 34(2): 131-136)
[0047] Take 2 kg of Epimedium sagittarius collected in the mountains of southern China, grind it into powder, and then extract it with 95% ethanol (10 L). 250 g of concentrated material were obtained, which was then further extracted with 2.5 L of chloroform, ethyl acetate and n-butanol. Pack 8 g of ethyl acetate extract and 10 g of n-butanol extract into a silica gel chromatography column, and wash with CHCl 3 -MeOH-HCOOH (15:1:0.5) and CHCl 3 -MeOH (8:2) eluted separately. Through this process, 300 mg of icariin and noricariin precursor icariin can be obtained. The precursor is then hydrolyzed according to the method briefly described below to obtain icariin. Solution A: 80 mg precursor was sonicated in 18 ml methanol solution; Solution B: 500 U (0.433 g) cellulase was dissolved in 180 ml (0.1 mol / L), pH 5.0 acetate buffer. Solution A was slowly a...
Embodiment 2
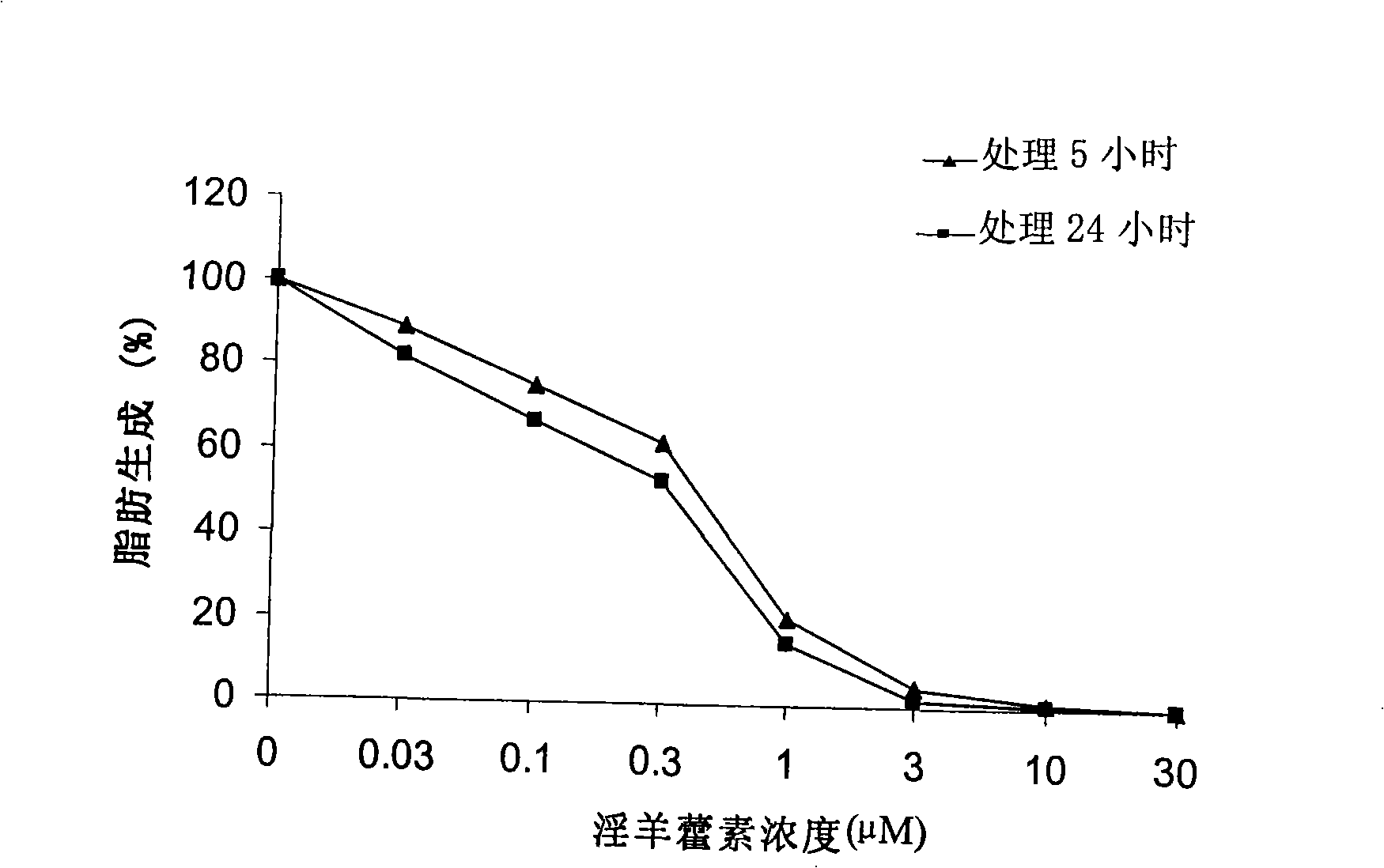
[0049] Use (2- 14 C) Inhibitory activity of icariin on cellular fatty acid production determined by acetate incorporation method
[0050] After treating LnCAP cells with different concentrations of icariin for 5 hours or 24 hours, 2- 14 C-labeled acetate (57 mCi / mmol; 2 μCi / dish; Amersham Biosciences) was added to the LnCAP cell culture medium. After incubation for 4 hours, collect the cells and medium, and resuspend the collected cells in 0.8ml PBS. Apply the Bligh Dyer method (Swinnen J.V. et al., Endocrinology 1996; 137:4468-4474) to extract lipids, and quantify the (2- 14 C) Acetate incorporation. The obtained results were normalized to the sample protein content. See the lipogenesis-icariin concentration curve after icariin treatment of LnCAP cells figure 1 .
Embodiment 3
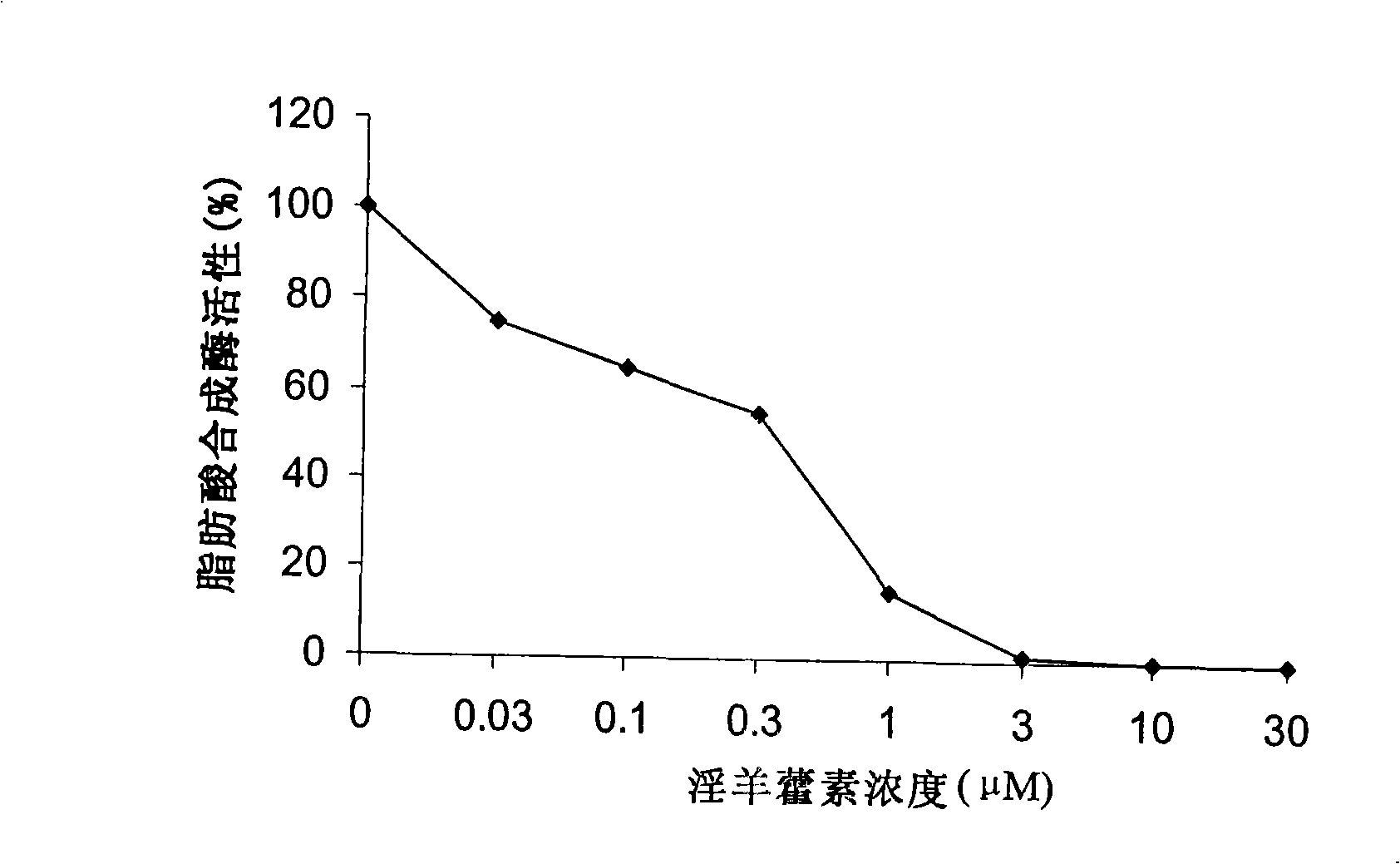
[0052] Inhibition of Icaritin on the Activity of Fatty Acid Synthase in LnCAP Cell Extracts
[0053] The FAS enzyme activity was determined using LnCAP cell protein extracts (Brusselmans K. et al., J Biol Chem. 2005 Feb 18; 280(7): 5636-5645). LnCAP cells were collected by centrifugation, resuspended in hypotonic buffer (1 mM EDTA, 20 mM Tris-HCl, pH 7.5), and then BCA method (Pierce) was used to determine the protein content of the sample. An equal amount of protein (50 μg) and different concentrations of icariin (0.03-30 μm) were placed in 2.5 ml of phosphate buffer (100 mM, pH 7.0), and pre-incubated at 37° C. for 30 minutes. Then add 20 μl reaction solution [2.5mM NADPH, 1.25mM acetyl-CoA, 1.25mM malonyl-CoA, 0.02mM [2- 14 C] Malonyl-CoA (60mCi / mmol; PerkinElmer Life Sciences)], incubate at 37°C for another 15 minutes, add 3ml of ice-cold 1M hydrochloric acid / methanol mixture (6:4, V / V) to terminate the reaction, and wash with petroleum Ether-extracted fatty acids were a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com