Medicinal preparation for resisting intracellular mycoplasma infection and application thereof
A technology for mycoplasma infection and pharmaceutical preparations, applied in the field of medicine, can solve the problems of cell recontamination, pollution, cell damage, etc., and achieve the effect of no loss of antibacterial effect, low toxicity and drug resistance, and small cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Adopt alkaline solution dissolution method to prepare sparfloxacin anti-mycoplasma intracellulare preparation, the steps are as follows: 4g sodium hydroxide (NaOH) is dissolved in 800ml ultrapure water, after fully dissolving, be settled to 1L, obtain concentration and be 0.1mol / l NaOH solution can be stored at room temperature, protected from light, and sealed for 6 months. NaOH is corrosive, and gloves are required for operation. Dissolve sparfloxacin technical powder fully in 0.1mol / l NaOH solution to obtain sparfloxacin anti-mycoplasma intracellulare preparation with a final concentration of 10 mg / ml, which is filtered through 0.45 μm and 0.22 μm filters successively and stored. Pharmaceutical preparations can be stored at -20° or -80° for long-term storage, and short-term (within 1 month) at 4° and protected from light to avoid repeated freezing and thawing.
Embodiment 2
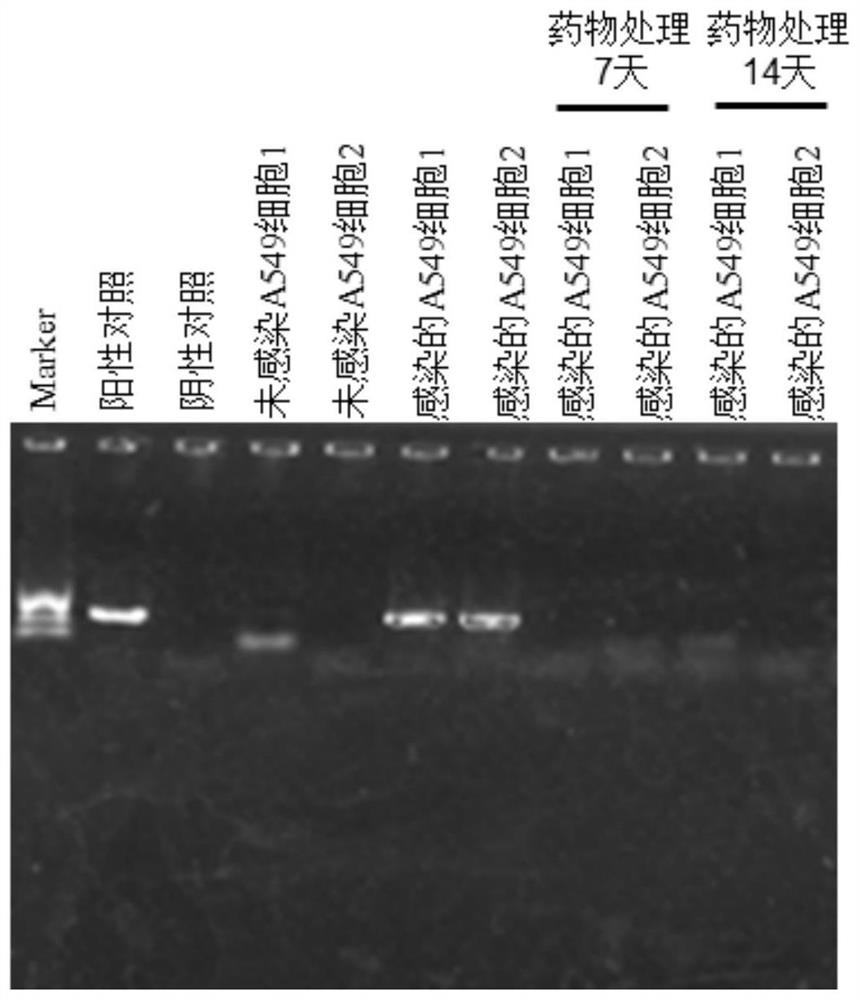
[0030] Verify that the sparfloxacin anti-intracellular mycoplasma preparation prepared in Example 1 has a clearing effect on intracellular mycoplasma, the steps are as follows: remove the original cell culture medium, replace it with a fresh medium after 3 times with PBS, and replace the above-mentioned sparzafloxacin Star anti-mycoplasma intracellular preparation was added to the culture medium at a ratio of 1:1000 (1 μl sparfloxacin anti-mycoplasma intracellular preparation was dissolved in 1 ml medium); the medium was changed every 1-2 days and continued to be used for 7 days.
[0031] In order to detect the ability of sparfloxacin anti-mycoplasma intracellular preparations to remove mycoplasma in cells, according to the document "Detection of Mycoplasma in cell cultures" (Nat Protoc.2010,5(5):929-34.doi:10.1038 / nprot. 2010.43) method application PCR is detected, and the method is as follows:
[0032] (1) Collect the culture medium of mycoplasma-contaminated cells and the c...
Embodiment 3
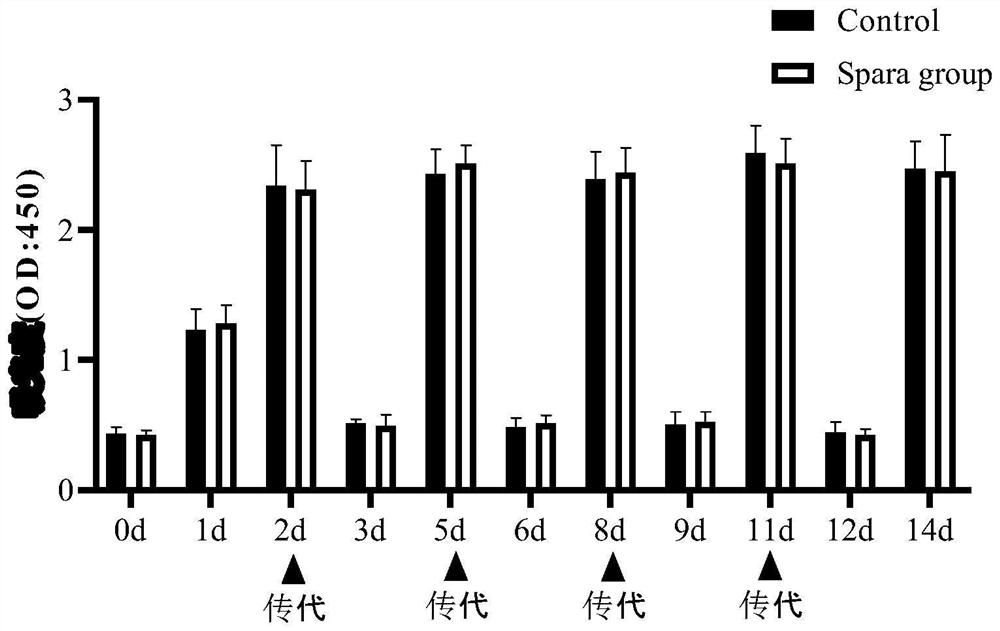
[0044] Verify that the sparfloxacin anti-mycoplasma intracellular preparation prepared in Example 1 has the effect on cytotoxicity, the steps are as follows: in the cell culture medium according to 1:1000 (1 μ l sparfloxacin anti-mycoplasma intracellular preparation is dissolved in 1 ml culture medium) Add the above-mentioned sparfloxacin anti-mycoplasma intracellular preparation, then pass the cells according to routine operations, and use CCK-8 to observe the cell viability and growth within 14 days. The results are as follows: figure 2 As shown, the growth status of A549 cells within 14 days was not affected.
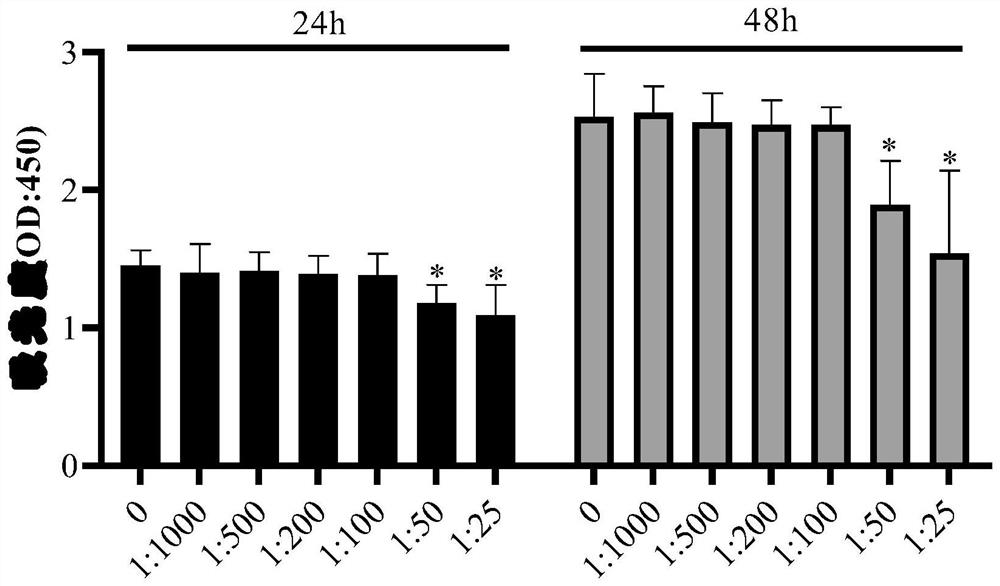
[0045] Test the impact of different concentrations of sparfloxacin anti-mycoplasma intracellular preparations on short-term cell survival, the steps are as follows: in the cell culture medium according to 1:1000 (1 μl sparfloxacin anti-mycoplasma intracellular preparations are dissolved in 1ml culture medium), 1:500, 1:200, 1:100, 1:50 and 1:25 were added to the abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com