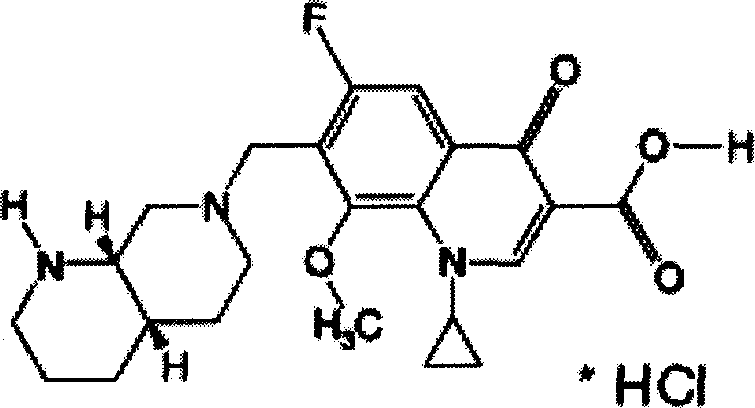
Moxifloxaci gelatin capsule and preparation process thereof
A technology of gelatin capsule and moxifloxacin, which is applied in the field of pharmaceutical preparation of moxifloxacin, can solve the problems of in vitro dissolution behavior change and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~17
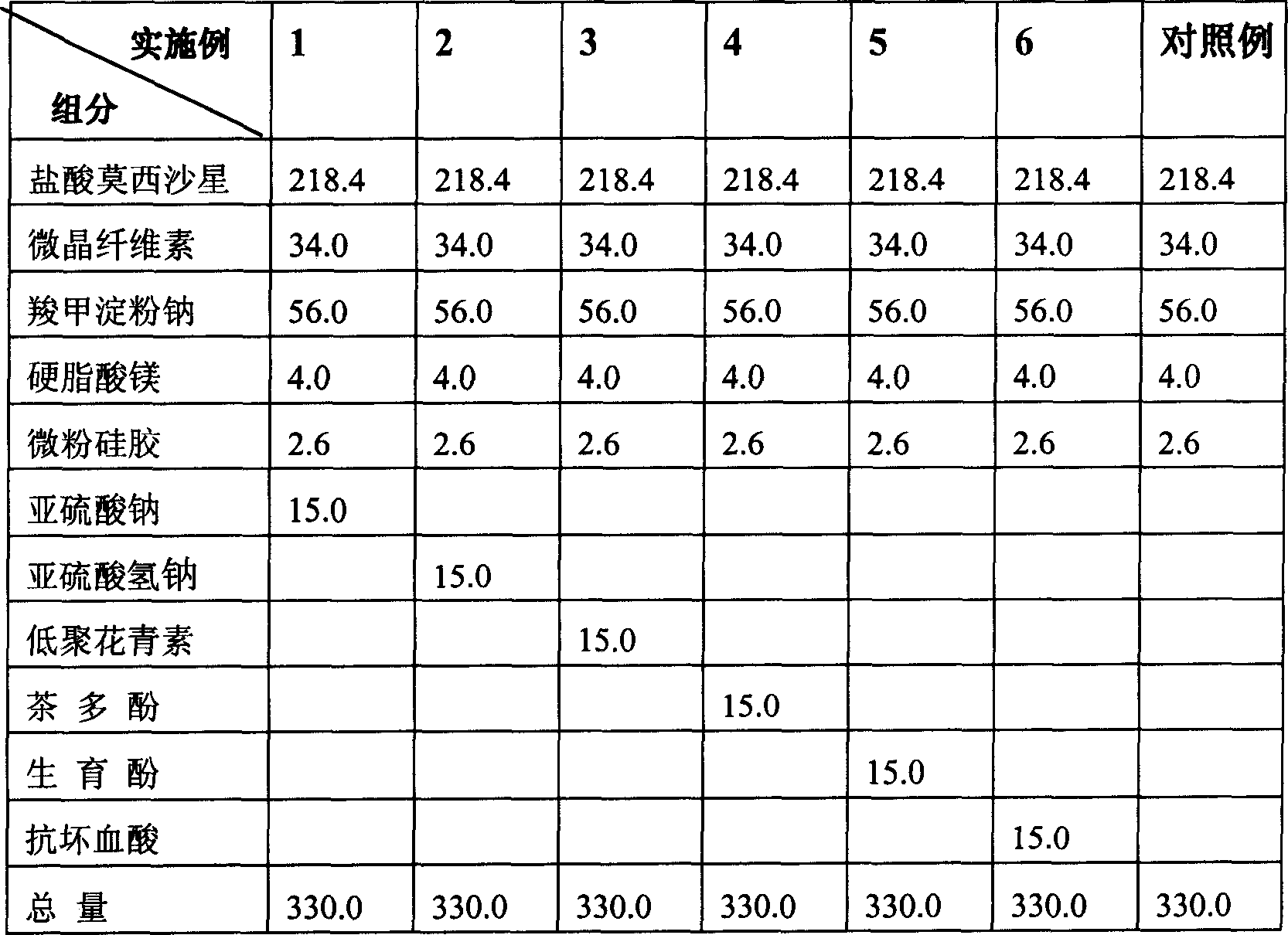
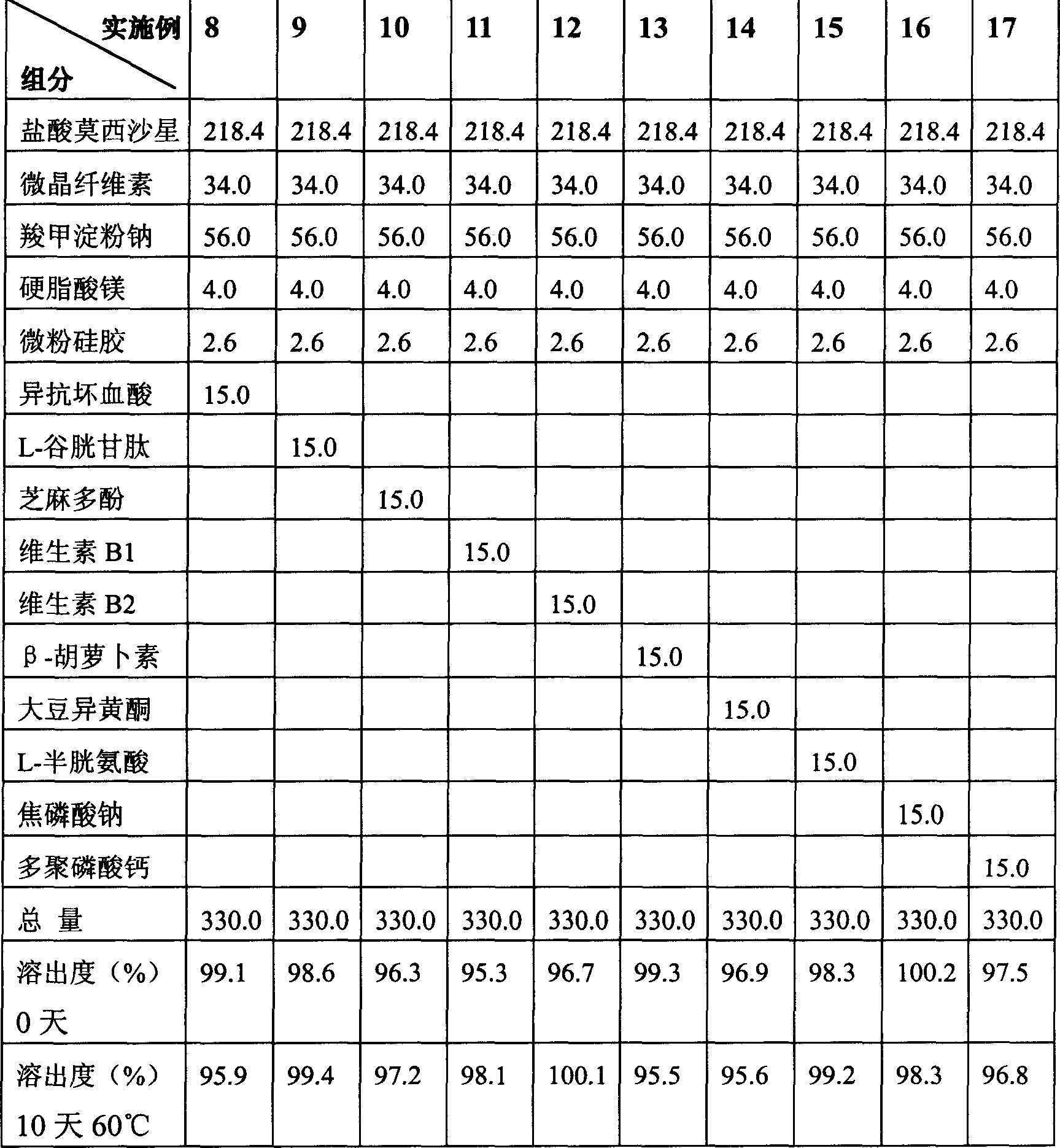
[0075] Embodiment 1~17 and comparative example
[0076] According to the dosage of each component listed in the following Table 1 and Table 2, sodium sulfite, sodium bisulfite, oligomeric anthocyanins, tea polyphenols, tocopherol, ascorbic acid, isoascorbic acid, L-glutathione, Sesame polyphenols, vitamin B1, vitamin B2, β-carotene, soybean isoflavones, L-cysteine, sodium pyrophosphate, and calcium polyphosphate were used as additives, and no additives (comparative example) were added to prepare molybdenum hydrochloride. Gelatin capsule of cifloxacin.
[0077] Preparation method and stability inspection: take the raw materials and auxiliary materials in the amounts stated in Table 1 and Table 2, mix them evenly, fill them into gelatin capsule shells, pack them in aluminum-plastic blisters, and investigate the dissolution rate after a 10-day stability test at 60°C .
[0078] The results show that after 10 days, the dissolution rate of the capsules added with the above-mention...
Embodiment 18~42
[0086] Composition: as shown in Table 3 to Table 8
[0087] Preparation method: take the amount of raw materials and auxiliary materials mentioned in the table, mix well, and fill the capsules.
[0088] Stability investigation: the capsules prepared above were put into a composite aluminum foil bag and sealed. After three months of accelerated stability investigation at 40°C and a relative humidity of 75%, the dissolution rate was investigated. The dissolution rate was measured according to the test example 1. method of determination.
[0089] The results showed that the dissolution rate was greater than 80%.
[0090] Table 3. (Amount of ingredients per capsule, in mg)
[0091]
[0092] Table 4 (the amount of components per capsule, in mg)
[0093]
[0094] Table 5 (the amount of components per capsule, in mg)
[0095]
[0096] Table 6 (the amount of ingredients per capsule, in mg)
[0097]
[0098] Table 7 (the amount of components per capsule, in mg)
[009...
Embodiment 43~47
[0102] Composition: as shown in Table 8
[0103] Preparation method: Take the amount of raw materials, auxiliary materials (except oligocyanidin), part of magnesium stearate, part of micropowder silica gel as stated in the table, mix well, dry granulate, add oligocyanidin and remaining stearic acid Magnesium, micronized silica gel, mix well, fill gelatin capsules.
[0104] Stability investigation: the capsules prepared above were put into a composite aluminum foil bag and sealed. After three months of accelerated stability investigation at 40°C and a relative humidity of 75%, the dissolution rate was investigated. The dissolution rate was measured according to the test example 1. method of determination.
[0105] The results showed that the dissolution rate was greater than 80%. The specific results are shown in Table 8
[0106] Table 8 (the amount of components per capsule, in mg)
[0107]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gross weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com