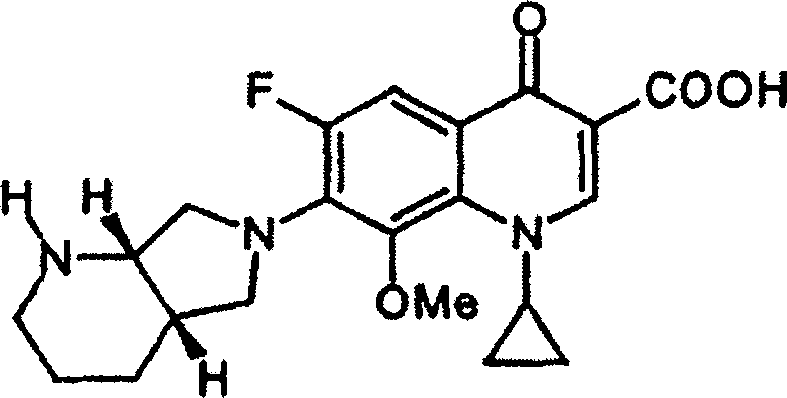
Freeze dry powdered injection of moxlfloxacin or its salts and preparation process thereof
A technology for freeze-dried powder injection and injection, applied in the field of freeze-dried powder injection of moxifloxacin or its salt for injection and its preparation, can solve the problems of poor solubility and inconvenience of moxifloxacin hydrochloride, and achieve long-term storage , Overcome the effects of drug decomposition and low water content in finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Then it is out of the box, plugged and capped, and the finished product is packaged after passing the inspection.
[0025] The obtained moxifloxacin freeze-dried preparation is a white lump with a loose texture, and each bottle contains 200 mg of the active drug moxifloxacin hydrochloride converted into moxifloxacin.
Embodiment 2
[0027] Under aseptic conditions, take moxifloxacin hydrochloride (converted into moxifloxacin) to be 10g, glucose 20g, take moxifloxacin hydrochloride of said weight, add 100ml of water for injection wherein, stir to make moxifloxacin hydrochloride completely Dissolve, add the glucose of the stated weight, stir to dissolve it completely, add 1mol / L sodium bicarbonate solution or sodium bicarbonate powder to adjust the pH value to 5.0, add water for injection until the total volume of the solution is 200mL, and use a 0.45μm micropore The filter membrane is initially filtered, and the filtrate is finely filtered with a 0.22um microporous membrane filter. Gained filtrate is divided into 5mL control antibiotic vials, each bottle is filled with 2ml, vacuum freeze-dried, and the freeze-drying process is the same as in Example 1. Then it is out of the box, plugged and capped, and the finished product is packaged after passing the inspection.
[0028] The obtained moxifloxacin freeze...
Embodiment 3
[0030] Under sterile conditions, weigh 5 g of moxifloxacin hydrochloride (converted into moxifloxacin) and 10 g of lactose, take the moxifloxacin hydrochloride of said weight, add 100 ml of water for injection to it, stir to make moxifloxacin hydrochloride completely dissolve , add the lactose of the stated weight, stir to dissolve it completely, add 1mol / L sodium bicarbonate solution or sodium bicarbonate powder to adjust the pH value to 5.3, add water for injection until the total volume of the solution is 200mL, and filter through a 0.45μm microporous filter Membrane primary filtration, filtrate fine filtration with 0.22μm microporous membrane. Gained filtrate is divided into 5mL control antibiotic vials, each bottle is filled with 2ml, vacuum freeze-dried, and the freeze-drying process is the same as in Example 1. Then it is out of the box, plugged and capped, and the finished product is packaged after passing the inspection.
[0031] The obtained moxifloxacin freeze-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com