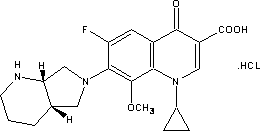
Preparation method for moxifloxacin and hydrochloride thereof
A technology of hydrochloride and boric acid, applied in the field of medicinal chemistry, can solve the problems such as the increase of impurities in the condensation reaction liquid, difficult drying, and chelation group shedding, so as to improve the yield and selectivity, ensure safety, and achieve zero discharge. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (a) Synthesis of borane chelates
[0034] Weigh 73.6g of acetic anhydride and add it to a 250ml four-neck flask, stir to raise the temperature to 90°C, add 13.6g of boric acid continuously, control the temperature of the reaction system at 90-95°C, keep the temperature at 90°C for 2 hours after the addition, cool down to 80°C, add 64.6 g mother nucleus (1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid ethyl ester) was stirred and heated to 105 °C, After 2 hours of reaction, the temperature was lowered to 60°C, and the reaction solution was slowly poured into 600ml of absolute ethanol, stirred to precipitate solids, filtered, and the filter cake was washed with 120ml of ice ethanol, dried and weighed: 80.7g, yield 95.5%.
[0035] (b) Synthesis of borane condensates
[0036] Add 80.7g of the chelate compound prepared in the previous step into a 500ml three-necked flask, then add 39.5g of potassium carbonate and 300ml of acetonitrile, stir...
Embodiment 2
[0042] (a) preparation of borane chelate
[0043] Weigh 73.6g of acetic anhydride and add it to a 250ml four-neck flask, stir to raise the temperature to 90°C, add 13.6g of boric acid in batches, control the temperature of the reaction system to stabilize at 90-100°C, keep the temperature at 90°C for 3 hours after the addition, and cool down after the reaction To 70°C, add 64.6g mother core, stir and raise the temperature to 105°C, react for 1.5h and cool down to 50°C, slowly pour the reaction solution into 600ml of isopropanol and vigorously stir to precipitate a solid, suction filter, filter cake with 120ml of ice isopropanol Alcohol washing, drying and weighing: 81.0g, yield 95.7%.
[0044] (b) Synthesis of borane condensates
[0045] Add 81.0g of the chelate compound prepared in the previous step into a 500ml three-necked flask, then add 52.8g of potassium carbonate and 300ml of acetonitrile, stir at 30°C, weigh 26.0g of the side chain, dilute with 50ml of acetonitrile an...
Embodiment 3
[0051] (a) Synthesis of borane chelates
[0052] Weigh 73.6g of acetic anhydride and add it to a 250ml four-neck flask, stir and heat up to 90°C, add 13.6g of boric acid in batches to ensure that the temperature is stable at 95-100°C, keep warm at 90°C for 2 hours after the addition, and cool down to 75°C after the reaction is complete. ℃, add 64.6g mother core and stir to raise the temperature to 110℃, react for 1h and cool down to 70℃, slowly pour the reaction solution into 600ml ethyl acetate and vigorously stir to precipitate solid, filter, wash the filter cake with 120ml glacial ethyl acetate, dry Dry weight: 80.0g. Yield 94.6%.
[0053] (b) Synthesis of borane condensates
[0054] Add 80.0g of the chelate compound prepared in the previous step into a 500ml three-necked flask, then add 78.3g of potassium carbonate and 300ml of acetonitrile, stir at 45°C, weigh 25.7g of the side chain and dilute it with 50ml of acetonitrile, and add it dropwise to the three-necked flask ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com