Method for purifying abiraterone acetate
A technology of abiraterone acetate and a purification method is applied in the field of purification of abiraterone acetate to achieve the effects of simplifying purification process, simple operation and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] For the preparation of crude abiraterone acetate, please refer to patents WO9509178, CN102030798, WO 2006021777, WO 2006021777, WO2006021776, J.Med.Chem.38, 2463-2471, 1995, or refer to patent GB 2282377, Org.Prep.Proced.Int, 1997, 29(l), 123-134, Prep.
[0034] For the salt formation of abiraterone acetate, please refer to the preparation method of WO2006021777 to obtain abiraterone acetate mesylate, abiraterone acetate hydrochloride and abiraterone acetate sulfate; refer to the preparation method of CN201010597372 to obtain abiraterone acetate trifluoromethane Sulfonate; refer to the preparation method of CN201110318824 to obtain abiraterone acetate oxalate, refer to CN201210039644 to obtain abiraterone acetate trifluoroacetate, and refer to CN201210201917 to obtain abiraterone acetate phosphate. These salt-forming methods can all obtain abiraterone acetate salt with better purity.
Embodiment 1
[0035] The selection of the acid used for the salification of abiraterone acetate in embodiment 1
[0036] In WO9320097, each step of column chromatography is used for purification. Since industrial production cannot be realized, some documents (WO2006021776, CN101044155, etc.) mention that the purification can be carried out by salt formation and crystallization, and then the product can be obtained by desalting. With reference to the description of CN102030798, we selected several acids, investigated and selected a suitable acid through the yield of salt formation and the situation of the thin layer chromatography (TLC) plate.
[0037] Table 1 Acid selection
[0038] acid Salt character Yield% oxalic acid Abiraterone Acetate Oxalate White solid 49.7% phosphoric acid Abiraterone Acetate Phosphate pale yellow solid 50.9% Methanesulfonic acid Abiraterone Acetate Mesylate yellow solid 52.3 Trifluoromethanesulfonic acid Abirate...
Embodiment 2
[0040] The preparation of embodiment 2 abiraterone acetate hydrochloride
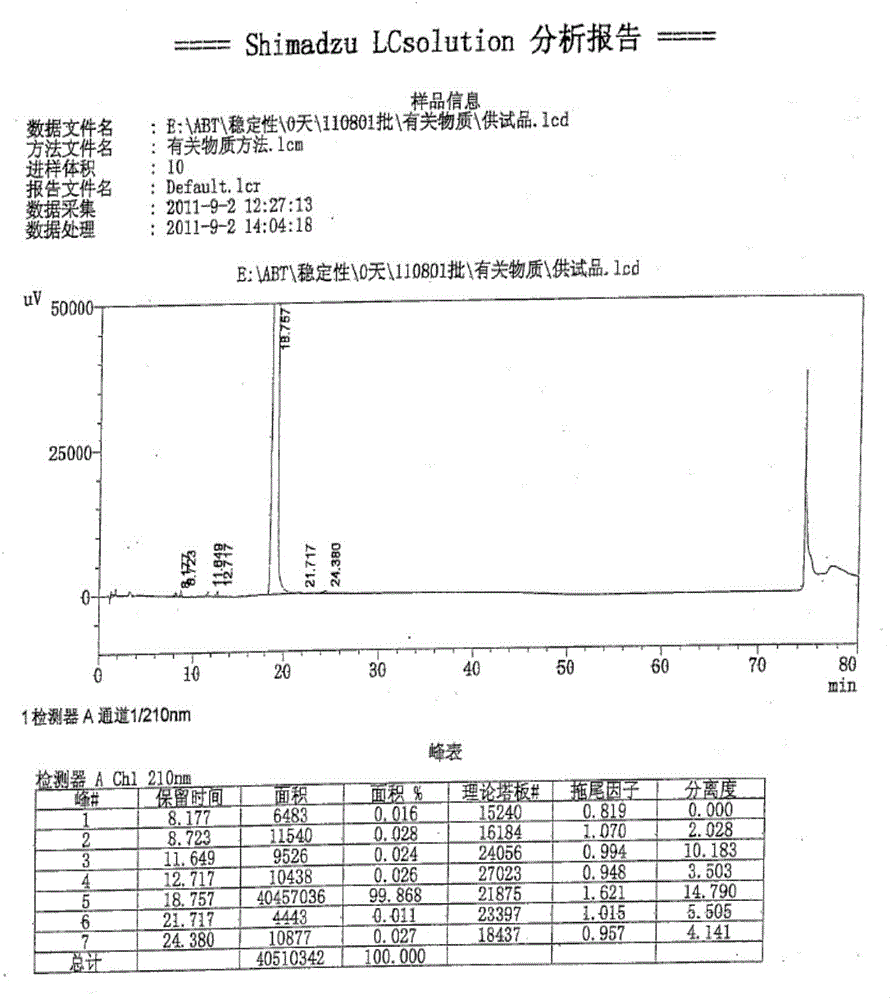
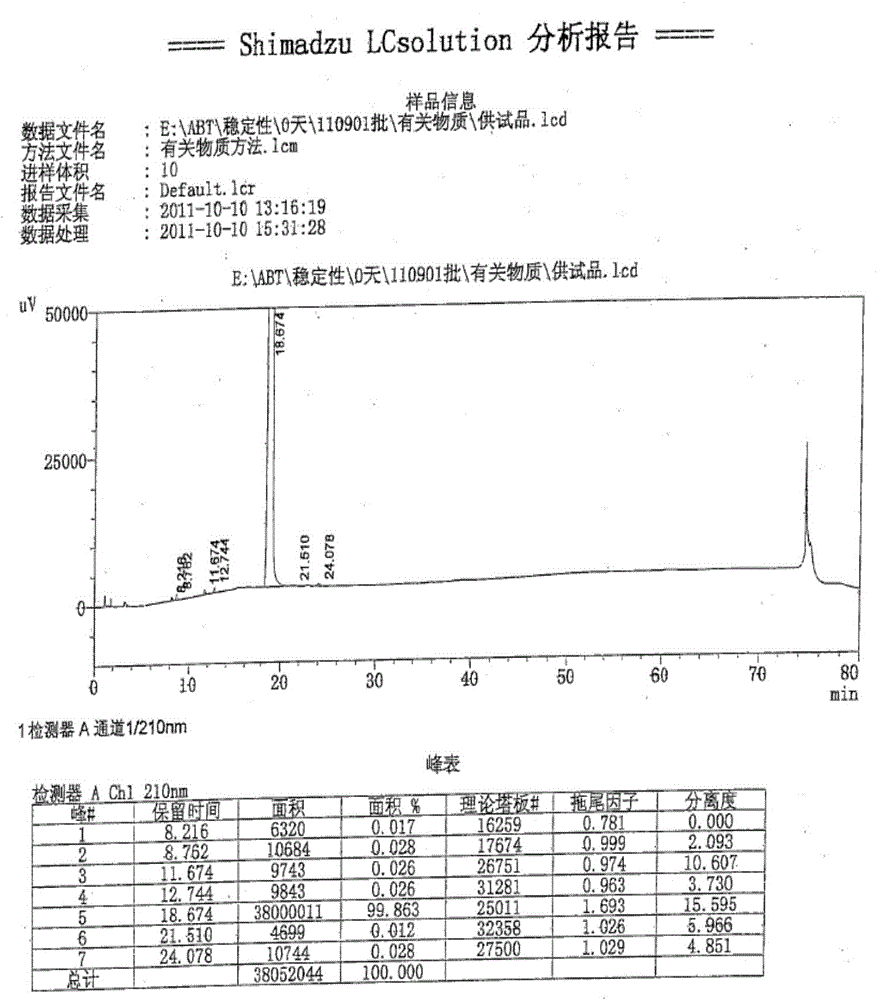
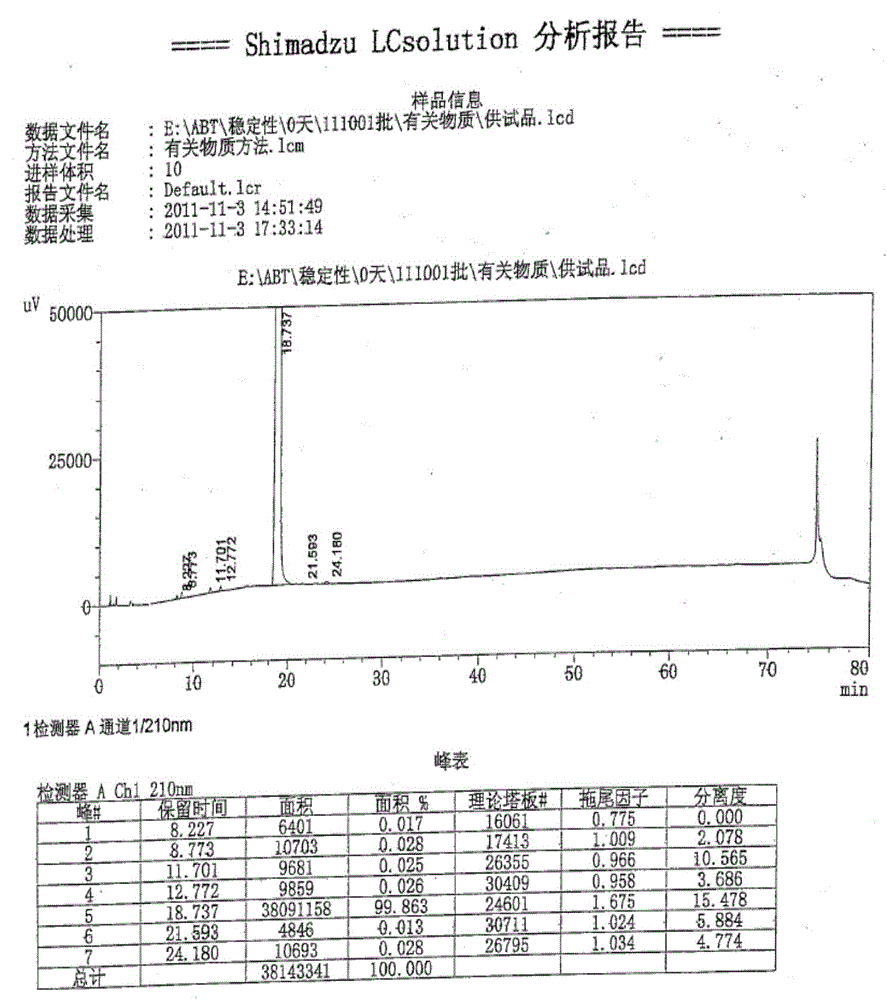
[0041] Add 2.6 kg of crude abiraterone acetate, 11 L of ethyl acetate and 11 L of methyl tert-butyl ether into the reaction kettle, and stir to dissolve. Stir and cool down to 5°C, and add 0.22 kg of concentrated hydrochloric acid dropwise. Stir and crystallize at 0~5°C for 2h. After warming up to room temperature, stir for 1 hour, filter with suction, and rinse the filter cake with methyl tert-butyl ether to obtain a yellow solid. The filter cake was air-dried at 35-40°C for 4 hours to obtain 2.5 kg of off-white solid. Yield: 86.7%, HPLC: 98.3%.
[0042] The high-performance liquid chromatography (HPLC) condition that purity detection is used: get sample appropriate amount, accurately weighed, add acetonitrile to dissolve and dilute to make the solution that contains about 3mg in every 1ml, as need testing solution; According to high-performance liquid chromatography (Chinese Pharmacopoeia 2010 edi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com