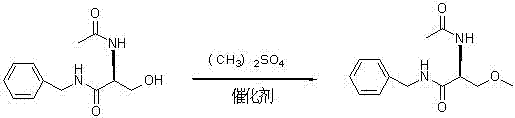
Synthetizing method of lacosamide
A technology of lacosamide and synthesis method, which is applied in the synthesis field of lacosamide, can solve the problems of low total yield, complicated operation and high cost, and achieves the effects of improving conversion rate, reducing reaction steps and short reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
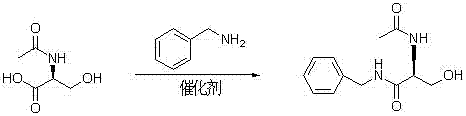
[0023] Acylation reaction
[0024] Add 150ml of dichloromethane to the reaction flask, add 10.5g of D-serine under stirring, cool to 10-15°C, add 11.3ml of acetic anhydride dropwise, maintain this temperature for about 20 minutes, and raise the temperature to 25-30°C for 1 hour. After the reaction is over, add 50ml of water, separate the organic phase, and use 8%Na 2 CO 3 Wash with 50ml, then wash twice with 50ml water, combine the organic phases, evaporate the solvent, add 100ml diethyl ether to disperse and stir, and precipitate a white solid, filter, and dry to obtain 14.2g (R)-2-acetylamino-3-hydroxypropane Acid (II).
Embodiment 2
[0026] Acylation reaction
[0027] Add 150ml of chloroform to the reaction bottle, add 10.5g of D-serine while stirring, add 14.2ml of acetic anhydride dropwise after cooling to 10-15°C, maintain this temperature for about 30 minutes, and raise the temperature to 25-30°C to react for 1.5 hours. After the reaction is over, add 50ml of water, separate the organic phase, and use 8%Na 2 CO 3 Wash twice with 30ml of water, then twice with 50ml of water, combine the organic phases, evaporate the solvent, then add 100ml of diethyl ether to disperse and stir, a white solid is precipitated, filtered, and dried to obtain 13.9g of II.
Embodiment 3
[0029] condensation reaction
[0030] Add 150ml of dichloromethane to the reaction bottle, add II 10.3g under stirring, after dissolving, add dropwise the prepared solution of 15.6g of DCC dissolved in 60ml of dichloromethane, react at 25-30°C for 0.5 hours, add benzylamine 7.5 g, react at 25-30°C for 3 hours, spot the plate to judge whether the reaction is complete. After the reaction, filter, wash the filtrate 3 times with 50ml water, combine the organic layers, concentrate to dryness, add 20ml chloroform-80ml methyl tert-butyl ether mixed solvent for recrystallization, and obtain (R)-2-acetylamino-3- Hydroxy-N-benzylpropionamide (Ⅲ) 12.4 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com