Method for producing recombinant human epidermal growth factor acceptor 2 cytoplasmic region with methylotrophy yeast
A methylotrophic yeast, using methyl technology, applied in the field of purification and identification, protein production, to achieve the effects of low production cost, stable expression system, and high expression yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
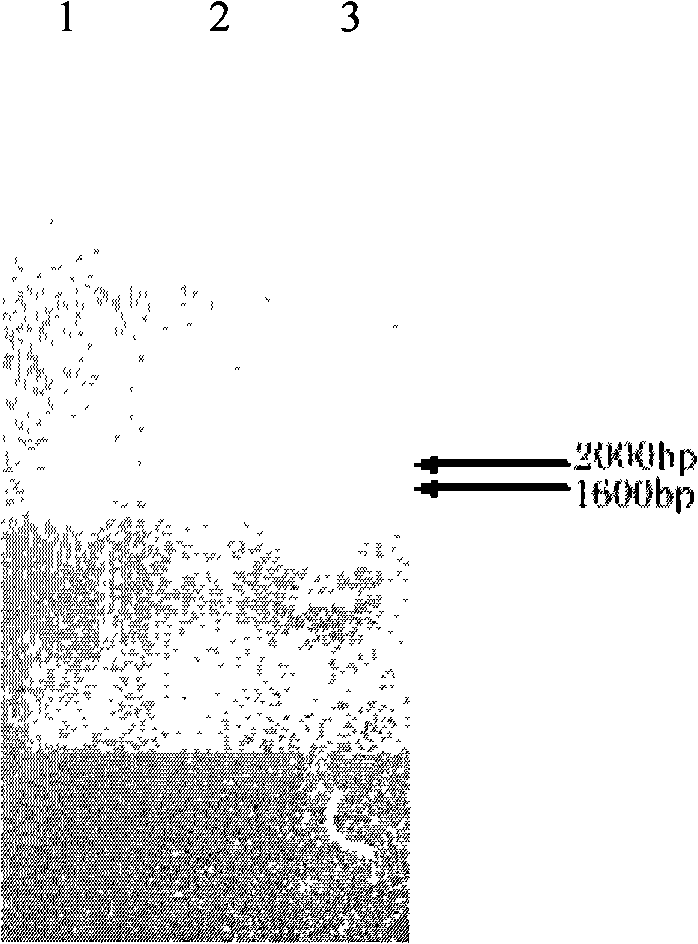
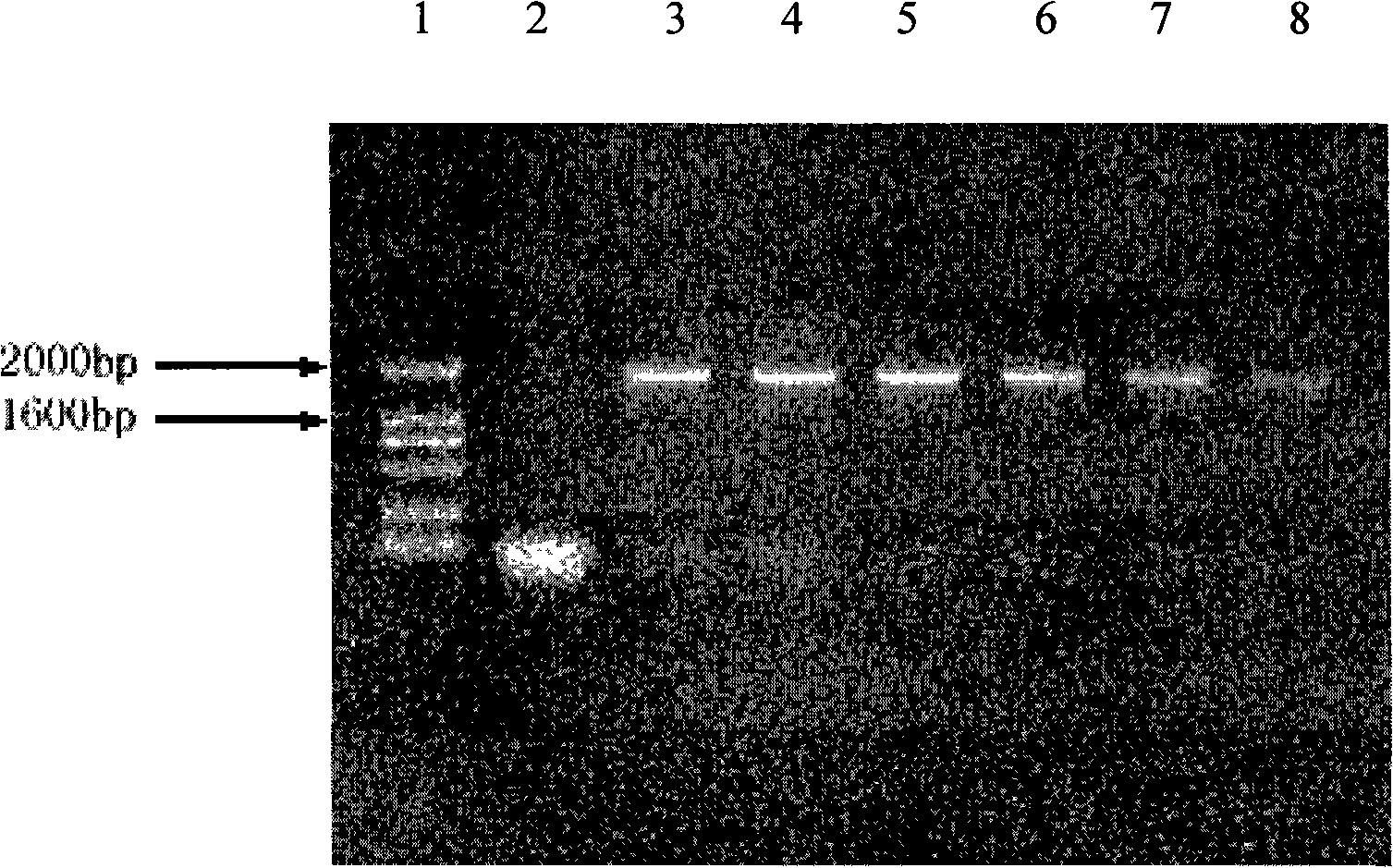
[0037] Example 1: Construction of rhHER2 / neu ICD Pichia secretory expression vector pPICZ α / her2 / neu ICD and host cell transformation
[0038] Using the plasmid pCMV (eukaryotic expression plasmid containing the full-length cDNA of human HER2 / neu) as a template, the her2 / neu ICD was cloned by PCR. The upstream primer: 5'-TTACTCGAGAAGAGAAAGCGACGGCAGCAG-3' (SEQ ID NO: 1) introduces the partial sequence of the yeast alpha factor signal peptide and the XhoI site, and uses the downstream primer: 5'-CTATCTTAGATCACACTGGCACGTCCAGACC-3' (SEQ ID NO: 2) An XbaI site is introduced. The PCR conditions were: denaturation at 94°C for 4 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 2.5 min; and extension at 72°C for 10 min. Perform 0.8% agarose gel electrophoresis analysis on the amplified product, observe the fragment size, and determine whether the correct target gene is obtained. After introducing the above sequences and restrictio...
Embodiment 2
[0043] Embodiment 2: Expression of HER2 / neu ICD protein
[0044] Inoculate the positive clones of the above identification results in 10ml of BMGY (pH6.0) medium, culture with shaking at 30°C for 24 hours, until OD 600 Cells were collected when reaching 2.0-6.0. Resuspend the cell pellet with an equal volume (10ml) of BMMY (pH6.0), culture with shaking at 30°C, and induce expression. During the induction process, methanol was supplemented every 24 hours to a final concentration of 0.5%, and sterilized ultrapure water was supplemented at the same time to keep the total volume of the fermentation broth unchanged. At the 0, 24, 48, 72, 96 and 120 hours of culture, 0.5 ml of fermentation broth was taken respectively, and the supernatant was collected by centrifugation for protein analysis (SDS-PAGE, Western Blot analysis, etc.).
Embodiment 3
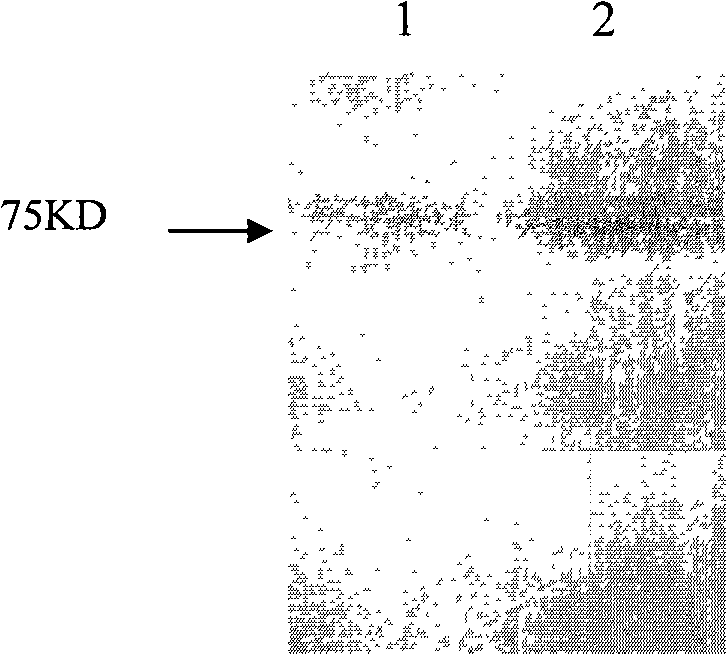
[0045] Example 3: Identification of physicochemical properties of HER2 / neu ICD protein
[0046] (1) SDS polyacrylamide gel electrophoresis (SDS-PAGE)
[0047] Protein molecular weight determination and preliminary quantitative analysis were carried out by SDS-PAGE method. Methods as below:
[0048] Prepare 10% separating gel and 5% stacking gel. Take the 48th hour culture supernatant and add 5×SDS sample buffer at a ratio of 4:1, mix well, and boil for 3-5 minutes. The above samples were taken, cooled to room temperature, centrifuged (10000r / min) for 30 seconds, and 20 μl of the supernatant was added to each well. 40V electrophoresis to the junction of the stacking gel and the separation gel, adjust the voltage to 100V, and continue the constant voltage electrophoresis separation. After staining with Coomassie Brilliant Blue and destaining, the analysis results were observed.
[0049] (2) Western blot analysis of expression products
[0050] According to conventional met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com