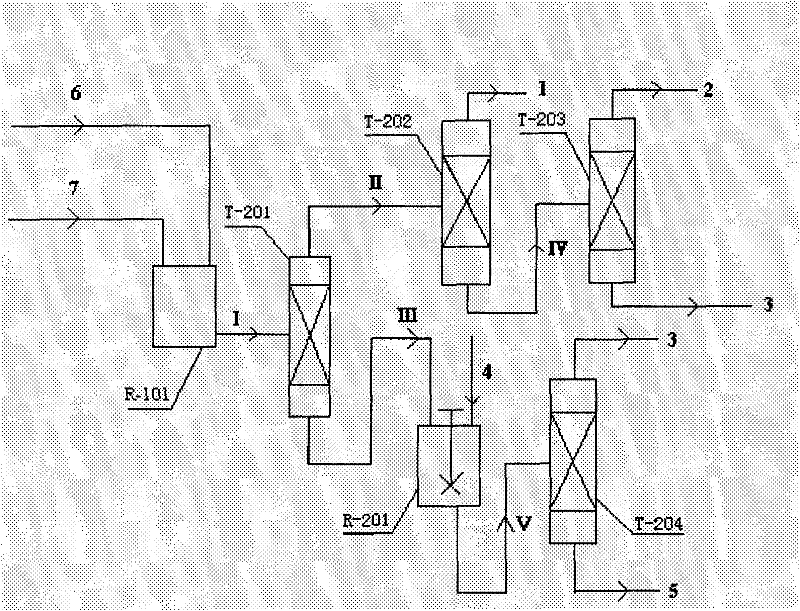
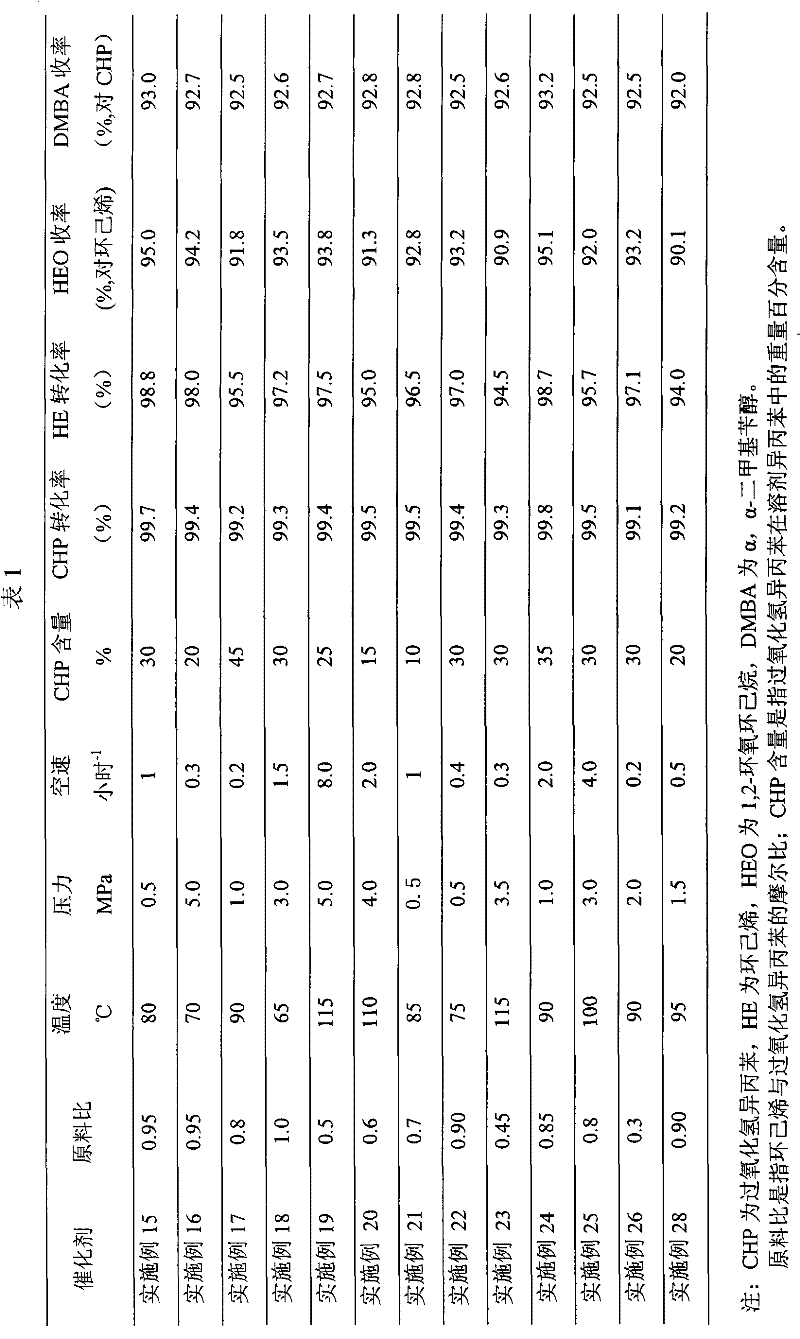
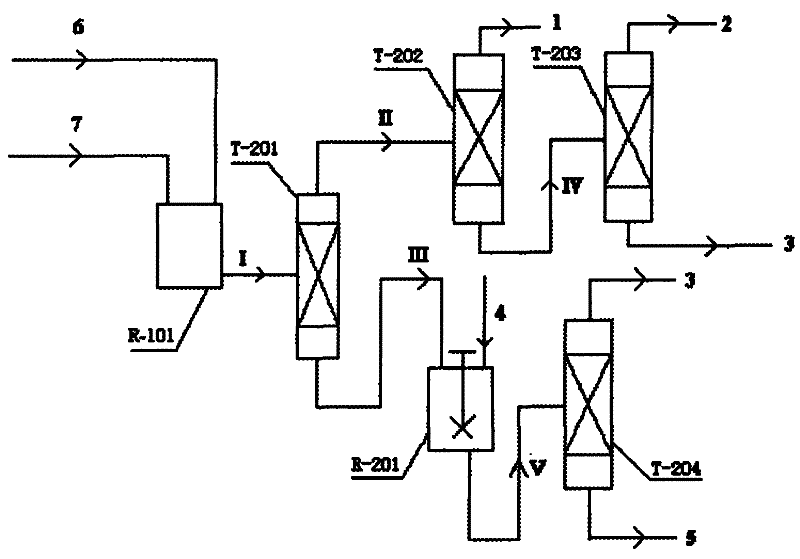
Coproduction method of 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol
A technology of dimethylbenzyl alcohol and epoxy cyclohexane is applied in the field of co-production of 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol, which can solve the pollution of production process and product quality. The problem of poor quality and high production cost can achieve the effect of good product quality, lower adsorption and lower acidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 30.0kg of fumed silica into 48.0kg of 25wt% tetramethylammonium hydroxide aqueous solution, and continuously stir for 30min to form a solution. Subsequently, the above solution was added to 120.0 L of aqueous solution containing 46.0 kg of cetyltrimethylammonium bromide under stirring to form a transparent solution. Transfer the above mixed solution into a crystallization kettle, add 0.1kg of MCM-41 as a seed crystal, and conduct static crystallization at 100°C for 3 days. The crystallized product was washed and filtered, dried at 100°C for 24 hours, and calcined at 550°C for 6 hours to obtain a catalyst carrier with the structural characteristics of MCM-41.
[0031] In the reactor, add 8.0kgTiCl 4 100L of cumene solution, the above-prepared catalyst support was added into the reaction kettle, under stirring and reflux, the temperature was raised to 150°C, and the reaction was carried out at this temperature for 4h. Then at this temperature, the residual TiCl was ...
Embodiment 2
[0034] Add 30.0kg of fumed silica into 48.0kg of 25wt% tetramethylammonium hydroxide aqueous solution, and continuously stir for 30min to form a solution. Subsequently, the above solution was added to 120.0 L of aqueous solution containing 46.0 kg of cetyltrimethylammonium bromide under stirring to form a transparent solution. Slowly add 4.8 kg of tetrabutyl titanate dropwise into the above mixed solution under rapid stirring and continue stirring for 30 min. Move the above mixed solution containing silicon and titanium into a crystallization kettle, add 0.1kg Ti-MCM-41 as a seed crystal, and statically crystallize at 100°C for 3 days to obtain the Ti-MCM-41 catalyst precursor.
[0035] According to the method of [Example 1], the prepared Ti-MCM-41 catalyst precursor is silanized, except that the silylating agent adopts 1,1,3,3-tetramethyldisilazane, and its consumption is 3.0kg , that is, the finished Ti-MCM-41 catalyst product treated with silane is obtained. XRD,N 2 Adso...
Embodiment 3
[0037] Ti-MCM-41 catalyst was prepared according to the method of [Example 1], except that the catalyst was not silanized. XRD,N 2 Adsorption, FT-IR and UV-Vis characterization and analysis results show that the material has typical MCM-41 structure characteristics and Ti has entered the framework to form four-coordinated active titanium, in which the weight content of titanium is 4.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com