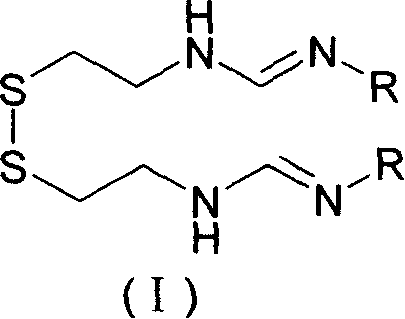
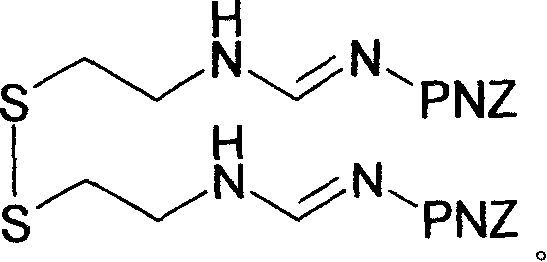
Imipenem intermediate and preparation method of imine peinan
A technology of imipenem and imipenem side chain, which is applied in the field of imipenem preparation, and can solve the problems of long synthetic route and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] [Example 1] N, N'-two [(N-p-nitrobenzyloxycarbonyl) imidoyl) cystamine N, the preparation of N'-Di(N-p-nitrobenzyloxycarbonyl) formimidoyl) cystamine:
[0035]
[0036] Under the protection of argon (or nitrogen) gas, 51.5 g (0.30 mol) of benzyl imidine ether hydrochloride and 100 ml of acetonitrile were added to form a suspension. A dry ice-ethanol bath cooled the reaction solution below -40°C, added 73.0g (98ml, 0.565mol) of diisopropylethylamine under stirring, then added 51.2g (0.238mol) of chloroformic acid at the same temperature to Nitrobenzyl ester is dissolved in the solution of 50ml acetonitrile, stirs 20min, the clear solution that obtains is the solution containing N-(p-nitrobenzyloxycarbonyl)-iminoformic acid benzyl ester (Benzyl (N-p-nitrobenzyloxycarbonyl) formimidate), as Reaction A.
[0037] In a 250ml single-necked bottle, add 26.7g (0.119mol) of cystamine dihydrochloride, then add 100ml of pyrrolidone and 31g (0.238mol) of diisopropylethylamine an...
Embodiment 2
[0044] [Example 2] N, N'-two [(N-benzyloxycarbonyl) iminomethylidene] cystamine N, the preparation of N'-Di((N-benzyloxycarbonyl) formimidoyl) cystamine:
[0045]
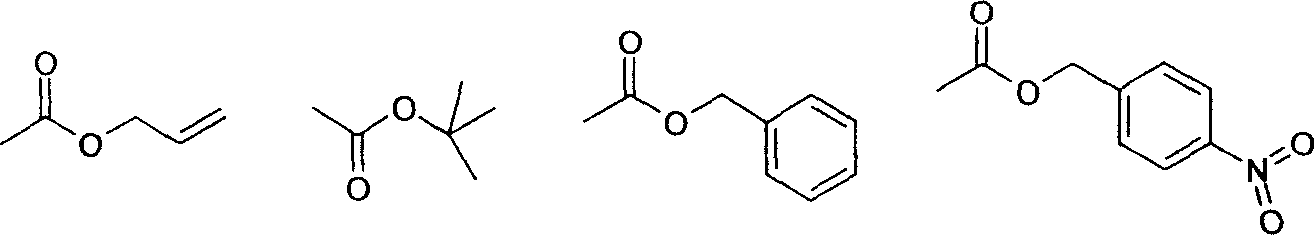
[0046] Except that the solution that the p-nitrobenzyl chloroformate that adds 51.2g (0.238mol) in embodiment 1 is dissolved in 50ml acetonitrile replaces the solution that the benzyl chloroformate that adds 40.6g (0.238mol) is dissolved in 50ml acetonitrile, other additions The amount and method are the same. Obtained N,N'-di[(N-benzyloxycarbonyl)iminomethylidene]cystamine [N,N'-Di((N-benzyloxycarbonyl)formimidoyl)cystamine]33.9g (yield 60%, content 96 %).
[0047] Mp: 120~122℃
[0048] Determination results of physical properties of the compound:
[0049] Ms: M+1=475
[0050] IR max KBr cm -1 : 1739, 1699, 1675, 1639, 1447, 1241, 1168, 761.
[0051] 1 H-NMR (δ, DMSO-d 6 ): 2.89 (4H, m, SCH 2 ), 3.55 (4H, m, NCH 2 ), 5.05 (4H, s, OCH 2 ), 7.34 (10H, m, ArH), 8.38 (2H, d, NCH=N).
[0052] 13 C-NMR...
Embodiment 3
[0053] [Example 3] N, N'-two [(N-allyloxycarbonyl) imidoyl] cystamine N, the preparation of N'-Di((N-allyloxycarbonyl) formimidoyl) cystamine:
[0054]
[0055] Except that the p-nitrobenzyl chloroformate that adds 51.2g (0.238mol) in embodiment 1 is dissolved in the solution of 50ml acetonitrile and replaces the solution that the allyl chloroformate of 28.7g (0.238mol) is dissolved in 50ml acetonitrile, other The dosage and method are the same. Obtained N, N'-bis[(N-allyloxycarbonyl)iminomethylidene]cystamine [N,N'-Di((N-allyloxycarbonyl)formimidoyl)cystamine] 33.4g (yield 75%, content 95%).
[0056] Mp: 108~110℃
[0057] Determination results of physical properties of the compound:
[0058] Ms: M+1=375
[0059] IR max KBr cm -1 : 1698, 1640, 1449, 1336, 1241, 1178, 1085, 983, 794.
[0060] 1 H-NMR (δ, DMSO-d 6 ): 2.91 (4H, m, SCH 2 ), 3.53 (4H, m, NCH 2 ), 4.50 (4H, d, OCH 2 ), 5.23 (4H, m, = CH 2 ), 5.91 (2H, m, -CH=), 8.35 (2H, d, NCH=N).
[0061] 13 C-N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com