Imipenem intermediate and preparation method thereof
A technology of imipenem and intermediates, applied in the fields of medicinal chemistry and chemical synthesis, can solve the problems of low conversion rate of reactants, poor crystallinity and the like, and achieve the effects of low cost, high yield and high crude product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
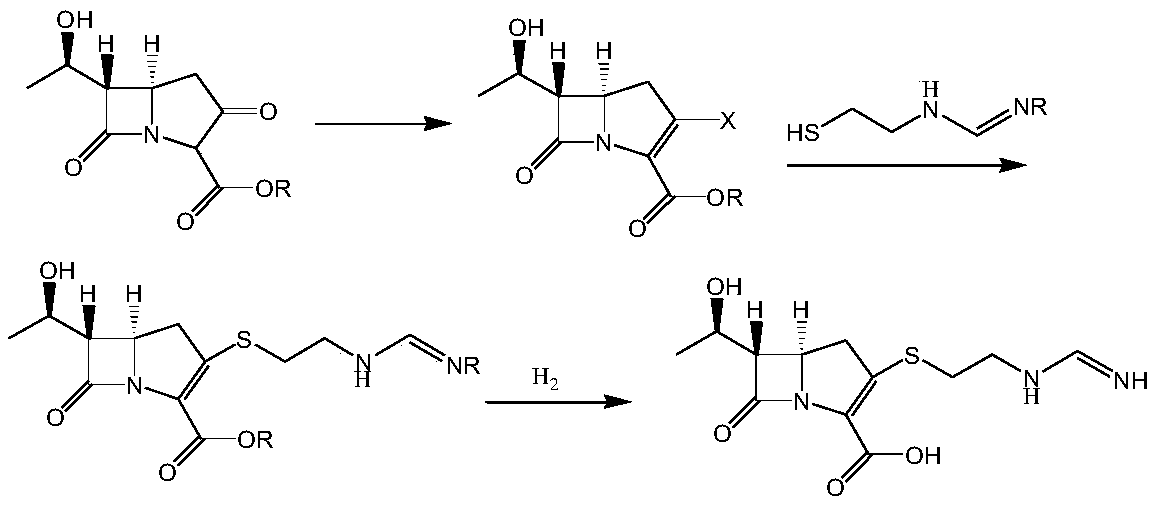
[0064] A preparation method of an imipenem intermediate, the method comprising the steps of:
[0065] (1) At -70 degrees Celsius, in the presence of an organic base N,N-diisopropylethylamine and under the protection of a nitrogen atmosphere, add compound III and diphenyl chlorophosphate to the organic solvent N-methylpyrrolidone in the reaction kettle Ester, obtains intermediate IV after thin-layer chromatography (TLC) detects that reaction is complete;
[0066] The ratio between the volume of the organic solvent N-methylpyrrolidone and the weight of compound III is 3ml:1g; the molar ratio of the organic base N,N-diisopropylethylamine to compound III is 1:1 ; The molar ratio of diphenyl chlorophosphate to compound III is 1:1;
[0067] The chemical structural formulas of the compound III and the intermediate IV are:
[0068]
[0069] R1 is p-nitrobenzyl or p-methoxybenzyl;
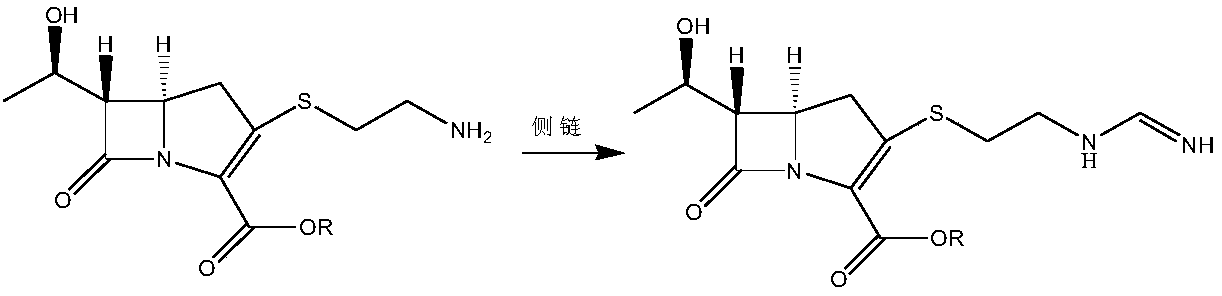
[0070] (2) At -50 degrees Celsius, in the presence of the organic base N,N-diisopropylethylamine, ...
Embodiment 2
[0093] (1) At 0 degrees Celsius, in the presence of an organic base N,N-dimethylaminopyridine and under the protection of a nitrogen atmosphere, compound III and diphenyl chlorophosphate were added to the organic solvent N-methylpyrrolidone in the reaction kettle, and the Intermediate Ⅳ was obtained after the reaction was completed by layer chromatography (TLC);
[0094] The ratio between the volume of the organic solvent N-methylpyrrolidone and the weight of the compound III is 15ml:1g; the molar ratio of the organic base N,N-dimethylaminopyridine to the compound III is 4:1; the The molar ratio of diphenyl chlorophosphate to compound III is 2:1;
[0095] The chemical structural formulas of the compound III and the intermediate IV are:
[0096]
[0097] R1 is p-nitrobenzyl or p-methoxybenzyl;
[0098] (2) At 0 degrees Celsius, in the presence of an organic base N, N-dimethylaminopyridine, add cysteamine hydrochloride to the intermediate IV, heat the reaction for 2.5 hours...
Embodiment 3
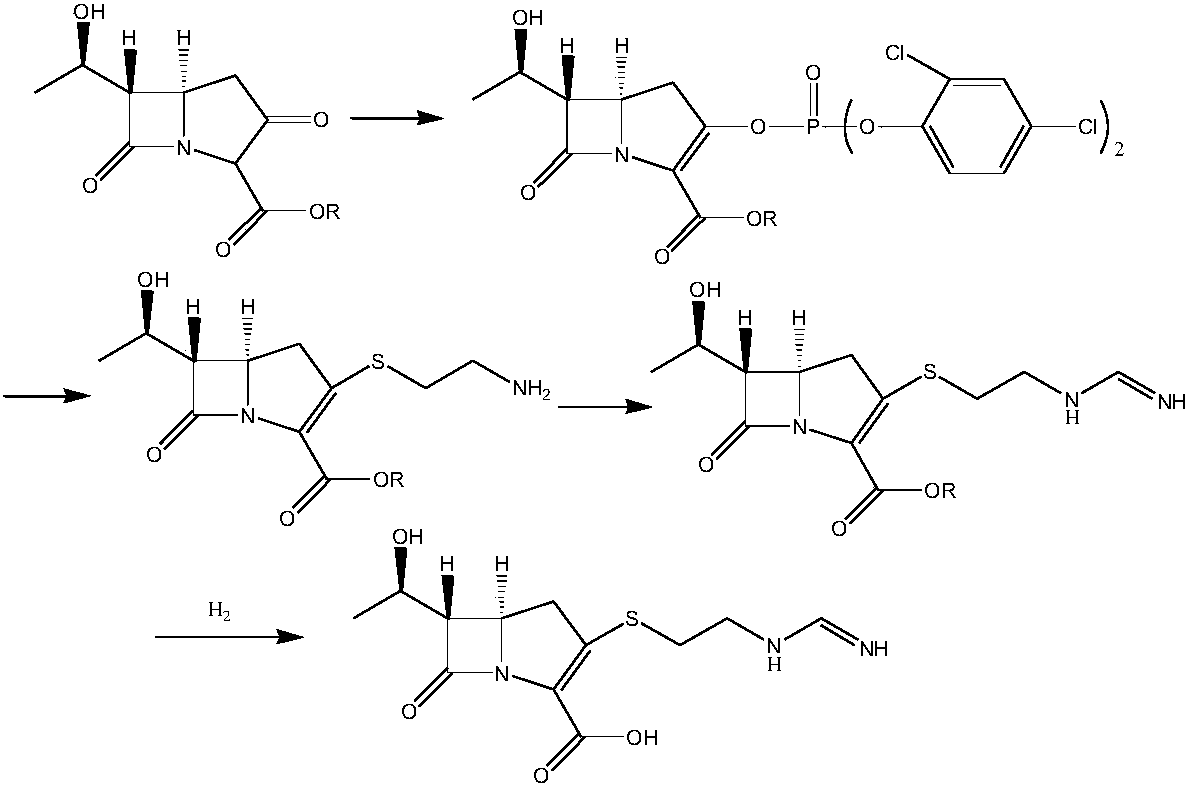
[0121] A preparation method of an imipenem intermediate, the method comprising the steps of:
[0122] (1) At -35 degrees Celsius, in the presence of organic base N,N-diisopropylethylamine and N,N-dimethylaminopyridine and under the protection of nitrogen atmosphere, the organic solvent N-methylpyrrolidone in the reaction kettle Add compound III and diphenyl chlorophosphate, and obtain intermediate IV after the reaction is complete by thin layer chromatography (TLC);
[0123] The ratio between the volume of the organic solvent N-methylpyrrolidone and the weight of compound III is 8ml:1g; the organic base N,N-diisopropylethylamine and N,N-dimethylaminopyridine and The molar ratio of compound III is 2:1; the molar ratio of diphenyl chlorophosphate to compound III is 1.2:1;
[0124] The chemical structural formulas of the compound III and the intermediate IV are:
[0125]
[0126] R1 is p-nitrobenzyl or p-methoxybenzyl;
[0127] (2) At -25 degrees Celsius, in the presence of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com