Preparation method of biquaternary ammonium salt compound
A salt compound and diquaternary ammonium technology, applied in the field of chemistry, can solve the problems of high cost of synthetic raw materials, complicated post-processing, etc., and achieve the effects of simple and economic post-processing and high-efficiency reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Reaction under different temperatures
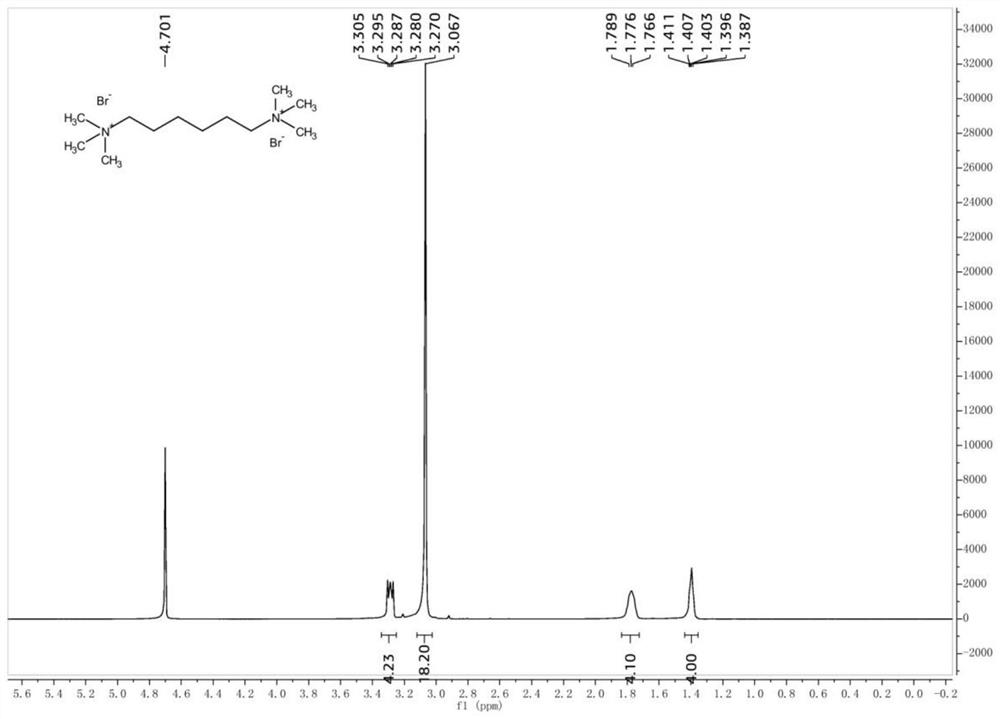
[0022] Take 1,6-dibromohexane (24.4g, 100mmol) and place it in a reaction kettle, add 50mL of ethanol, and mix well. Then trimethylamine aqueous solution (30%wt, 39.3g, 200mmol) was added, and the reaction was stirred at different temperatures for several hours. After the reaction, the solvent was evaporated under reduced pressure to obtain a white solid crude product. Add a small amount of ethyl acetate to the crude product, wash and filter with suction to obtain the product. The yields at different temperatures are shown in Table 1.
[0023] Table 1
[0024]
[0025]
[0026] It can be seen from Table 1 that within the same time period of 6 hours, the reaction yield at 25° C. is low, and a yield of 91.4% can be obtained by extending the reaction time to 12 hours. Heating the reaction solution to above 60°C can significantly increase the reaction yield. Increasing the reaction temperature from 60°C to 80°...
Embodiment 2
[0027] Embodiment 2: different molar ratio reaction
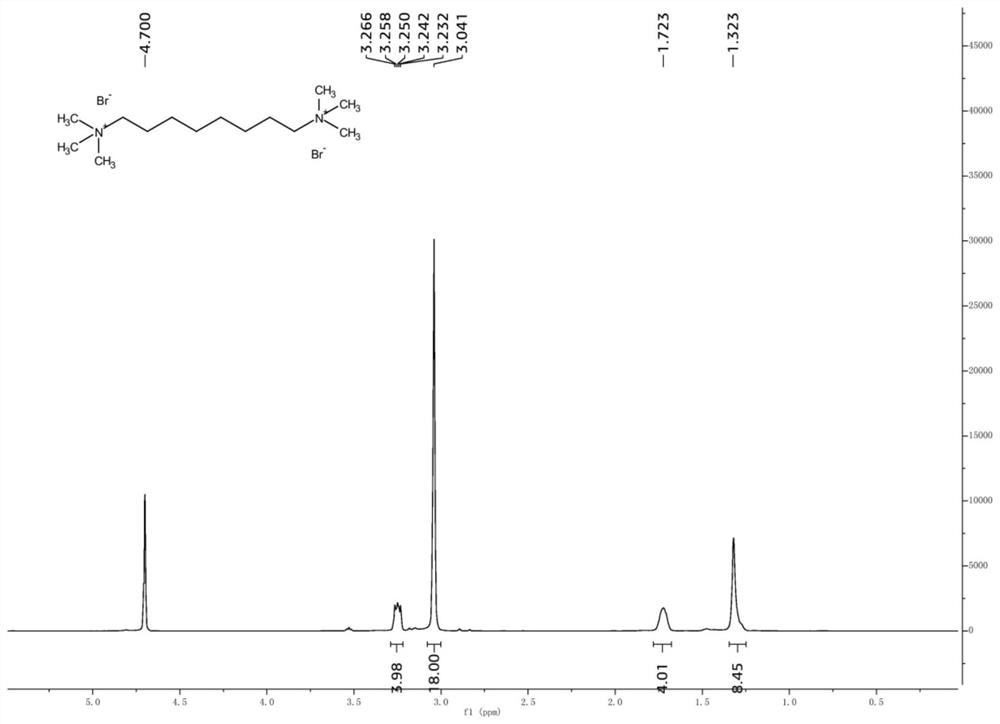
[0028] Take 1,6-dibromohexane (24.4g, 100mmol) and place it in a reaction kettle, add ethanol, and mix well. Then trimethylamine aqueous solution (30%wt) in different molar ratios was added, and the mixture was stirred and reacted at 60°C for 6 hours. After the reaction, the solvent was evaporated under reduced pressure to obtain a white solid crude product. Add a small amount of ethyl acetate to the crude product, wash and filter with suction to obtain the product. The reaction results of different molar ratios are shown in Table 2.
[0029] Table 2
[0030] serial number n(1,6-dibromohexane): n(trimethylamine) Yield (%) 1 1:2 95.5 2 1:2.2 96.9 3 1:2.4 96.5
[0031] It can be seen from Table 2 that, under the same other reaction conditions, increasing the trimethylamine to 2.2 equivalents can slightly increase the reaction yield, but continuing to increase the amount of trimethylamin...
Embodiment 3
[0032] Embodiment 3: washing with different washing solvents
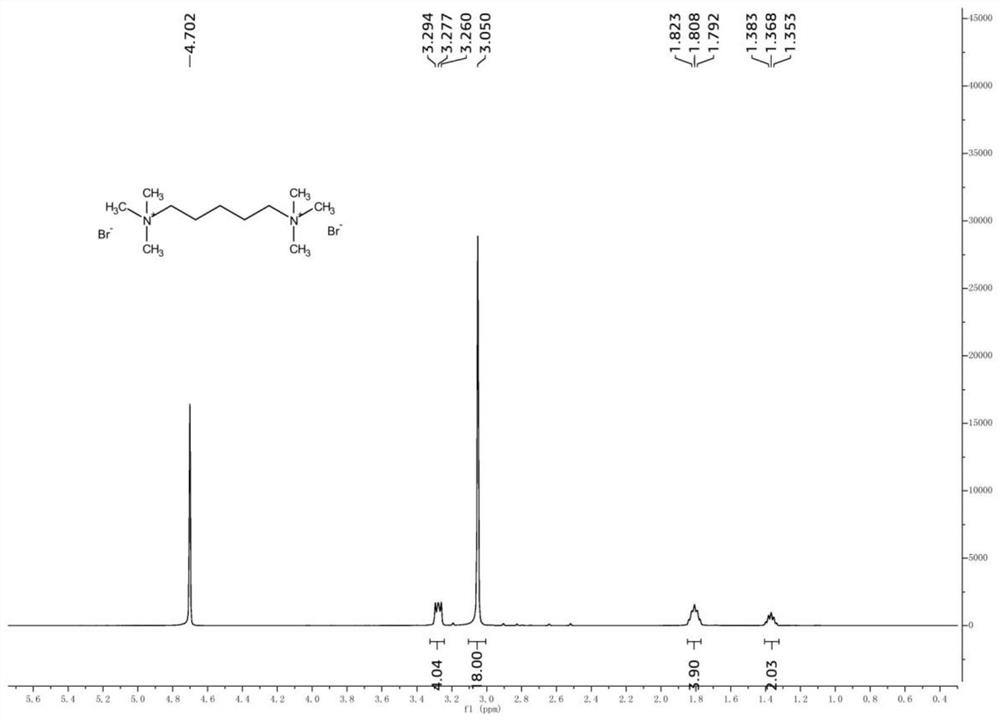
[0033] Take 1,6-dibromohexane (24.4g, 100mmol) and place it in a reaction kettle, add 50mL of ethanol, and mix well. Then trimethylamine aqueous solution (30%wt, 39.3g, 200mmol) was added, and the reaction was stirred at 60°C for 6 hours. After the reaction, the solvent was evaporated under reduced pressure to obtain a white solid crude product. Add a small amount of organic solvent to the crude product, wash and filter with suction to obtain the product. The yields after washing and suction filtration with different organic solvents are shown in Table 3.
[0034] table 3
[0035] serial number solvent Yield (%) 1 ethyl acetate 95.5 2 petroleum ether 96.3 3 Dichloromethane 96.0 4 ethanol 92.8
[0036] As can be seen from Table 3, different washing solvents lead to significant differences in yields, which are mainly related to the different solubility of bis-quaterna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com