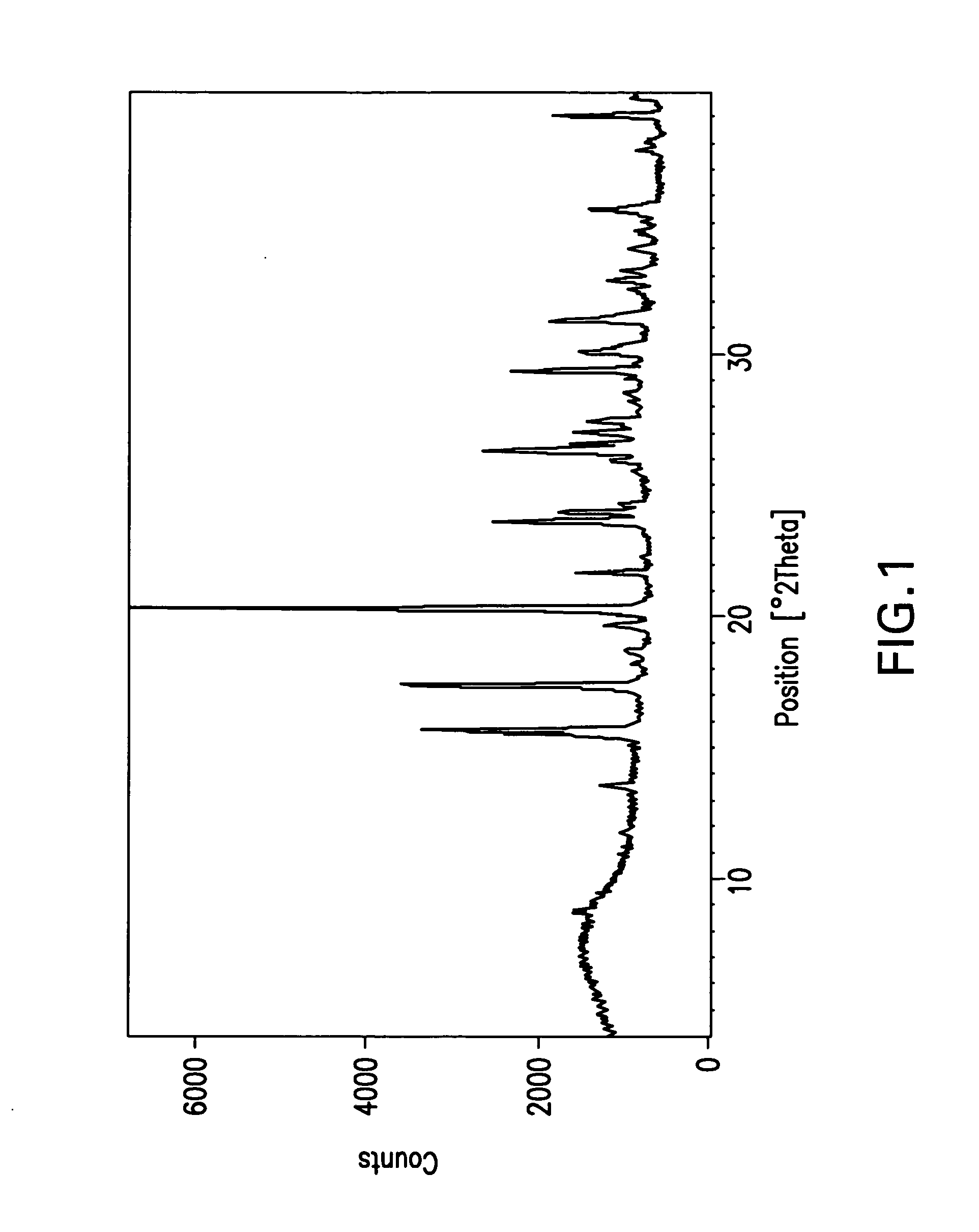
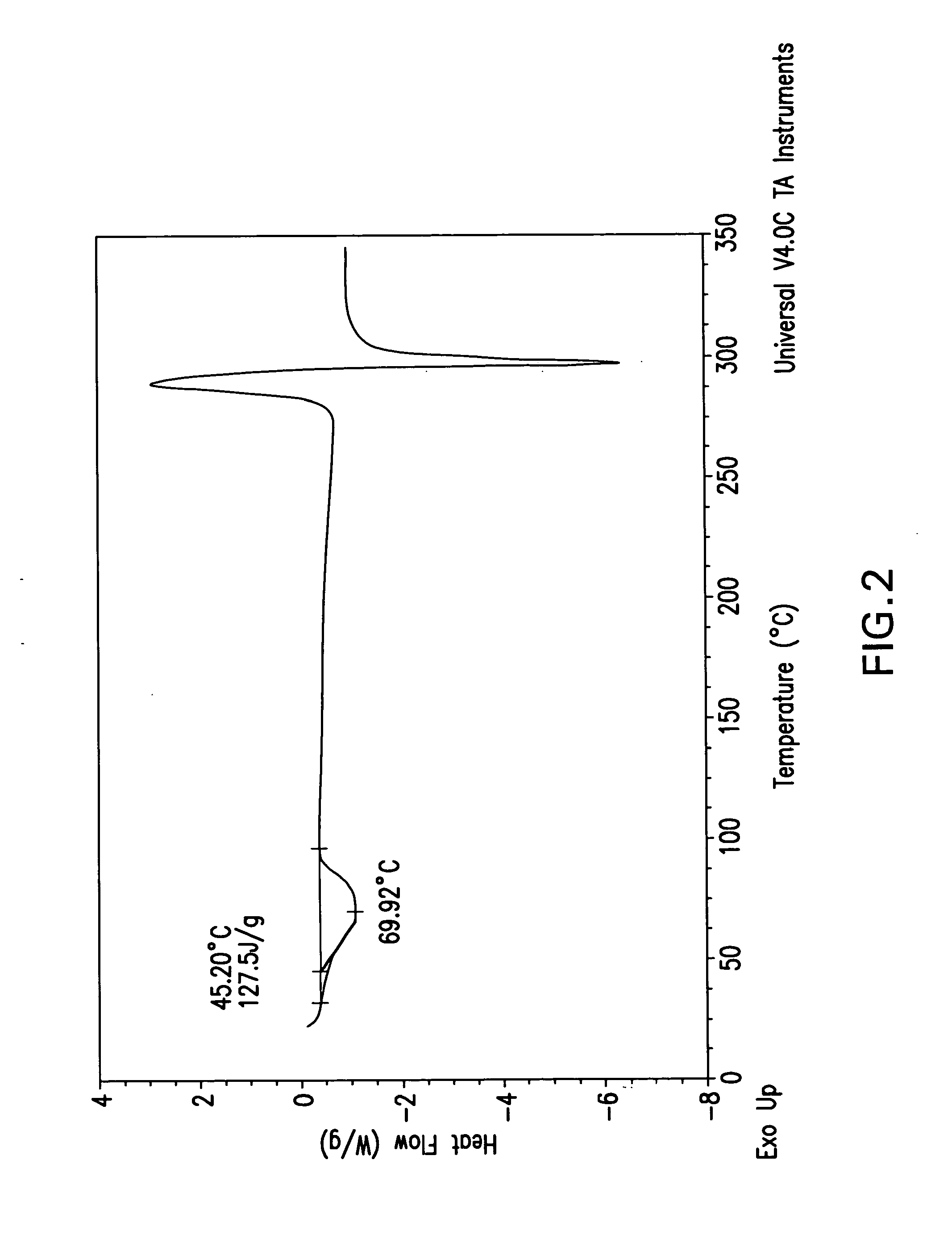
Beta-lactamase inhibitors
a technology of beta-lactamase and inhibitor, which is applied in the direction of biocide, antibacterial agents, drug compositions, etc., can solve the problems of increasing the cost of treatment, increasing the mortality of patients, and increasing the risk of infection, so as to improve the survival rate, inhibit beta-lactamase, and improve the effect of antibacterial
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
(2S,5R)-7-oxo-N-piperidin-4-yl-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
[0314]
Step 1: tert-butyl 4-({[(2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]oct-2-yl]carbonyl}amino)piperidine-1-carboxylate
[0315]To a solution of (2S,5R)-6-(phenylmethoxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxylic acid (0.141 g, 0.509 mmol) (note: this intermediate is disclosed in U.S. Pat. No. 7,112,592 Example 32b) in dry dichloromethane (3 mL) was added 4-amino-1-BOC-piperidine (0.1532 g, 0.765 mmol), triethylamine (0.16 mL, 1.148 mmol), HOBT (0.1145 g, 0.748 mmol), and EDC (0.1455 g, 0.759 mmol) sequentially at room temperature under nitrogen. The reaction was stirred at room temperature overnight. The reaction mixture was concentrated under vacuum and the residue was purified by HPLC (30×100 mm Waters Sunfire column; 5 micron; 35 mL / minute; 210 nM; 15% to 100% CH3CN+0.05% TFA / water+0.05% TFA over 15 minutes; desired product elutes at 50% CH3CN+0.05% TFA / water+0.05% TFA) to afford...
example 1a
[0319](2S,5R)-7-oxo-N-piperidin-4-yl-6-(sulthoxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
Step 1: tert-butyl 4-({[(2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]oct-2-yl]carbonyl}amino)piperidine-1-carboxylate
[0320]To a solution of (2S,5R)-6-(phenylmethoxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxylic acid (1.484 g, 5.37 mmol) in dry dichloromethane (60 ml) was added triethylamine (1.88 ml, 13.49 mmol), 2-chloro-1-methylpridinium iodide (1.60 g, 6.26 mmol), and 4-amino-1-BOC-piperidine (1.30 g, 6.49 mmol) sequentially at room temperature under nitrogen. The reaction was then heated to 50° C. for 1 hour. The reaction mixture was concentrated under vacuum and purified by silica gel chromatography on an Isco Combiflash (40 g silica gel, 40 mL / min, 254 nM, 15% to 100% EtOAc / hexane over 14 column volumes then 100% EtOAc for 4 column volumes; title compound eluted at 65% ethyl acetate / hexane) to afford the title compound as a pale orange solid.
Step 2: tert-butyl 4-({[(2S,5R)-6-...
example 1c
(2S,5R)-7-oxo-N-piperidin-4-yl-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
Step 1: Benzyl 4-[(tert-butoxycarbonyl)amino]piperidine-1-carboxylate
[0324]
[0325]4-(N-BOC amino) piperidine (17 kg, 84.88 mol) was dissolved in DCM (90 kg), triethylamine (10.14 kg, 100.16 mol) was added, and the resulting solution was cooled to 0-5° C. Benzyl chloroformate (16.51 kg, 96.76 mol) was added over 45 minutes while keeping the temperature at 1H NMR (CDCl3) 7.33 (5H, m), 5.13 (2H, s), 4.47 (1H, m), 4.11 (2H, m), 3.61 (1H, m), 2.93 (2H, m), 1.94 (2H, m), 1.45 (9H, s) and 1.30 (2H, m).
Step 2: Benzyl 4-aminopiperidine-1-carboxylate
[0326]
[0327]4-(N-BOC amino)-CBz piperidine (24.4 kg 73.42 mol), THF (65 kg) and 5 M HCl (23.0 kg, 110.13 mol) were combined and heated to 30-35° C. for ˜2 hours, and then at 55° C. overnight. After cooling the reaction mixture to 10° C., dichloromethane (97 kg) and 10M NaOH (7.97 kg, 145.12 mol) were added, while keeping the temperature at 1H NMR (CDCl3) 7.33 (5H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com