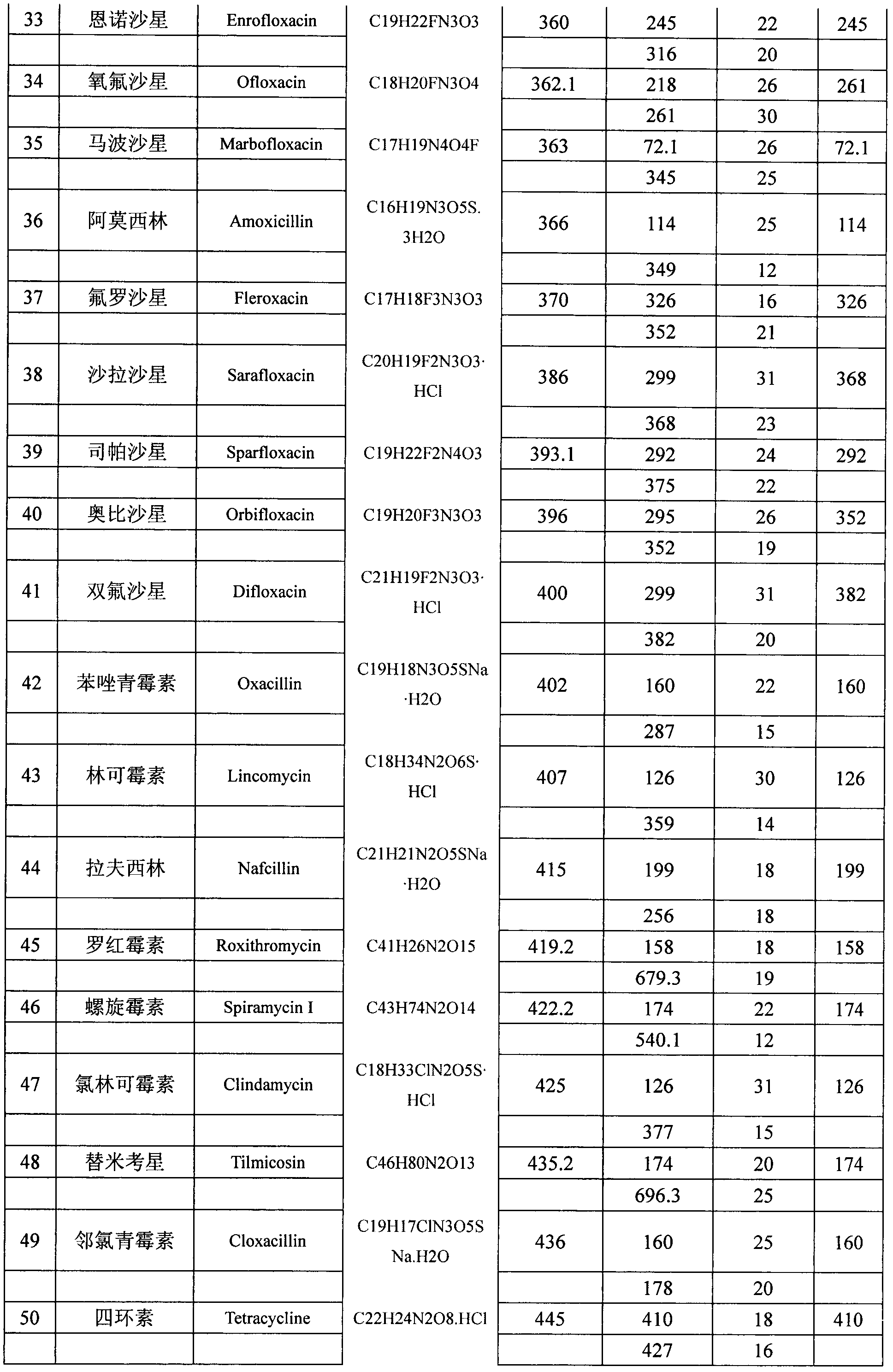
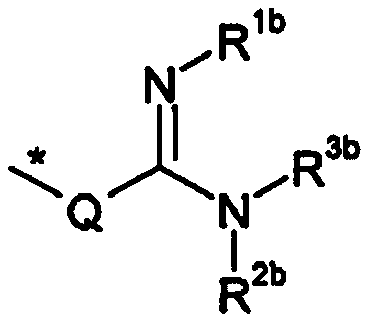
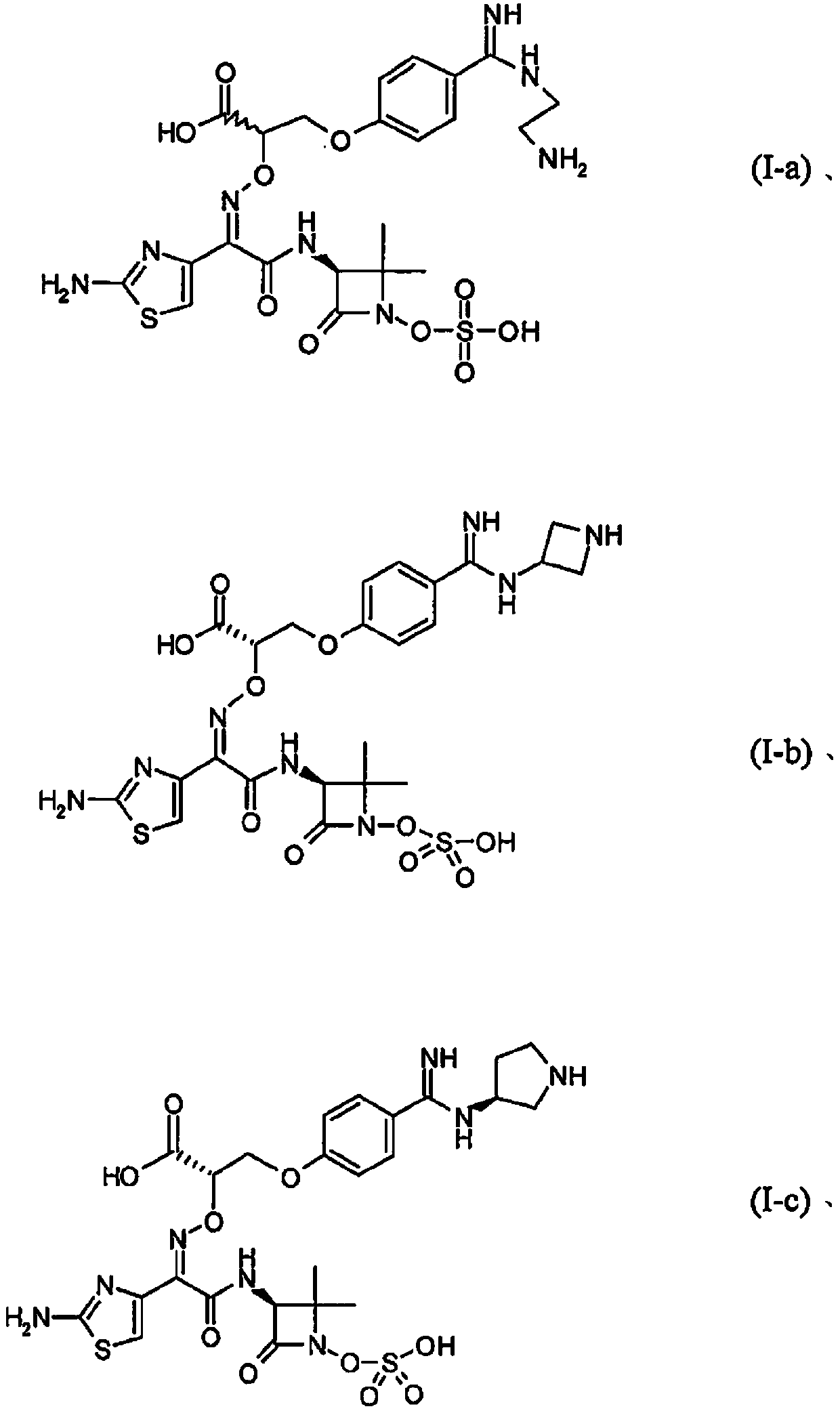
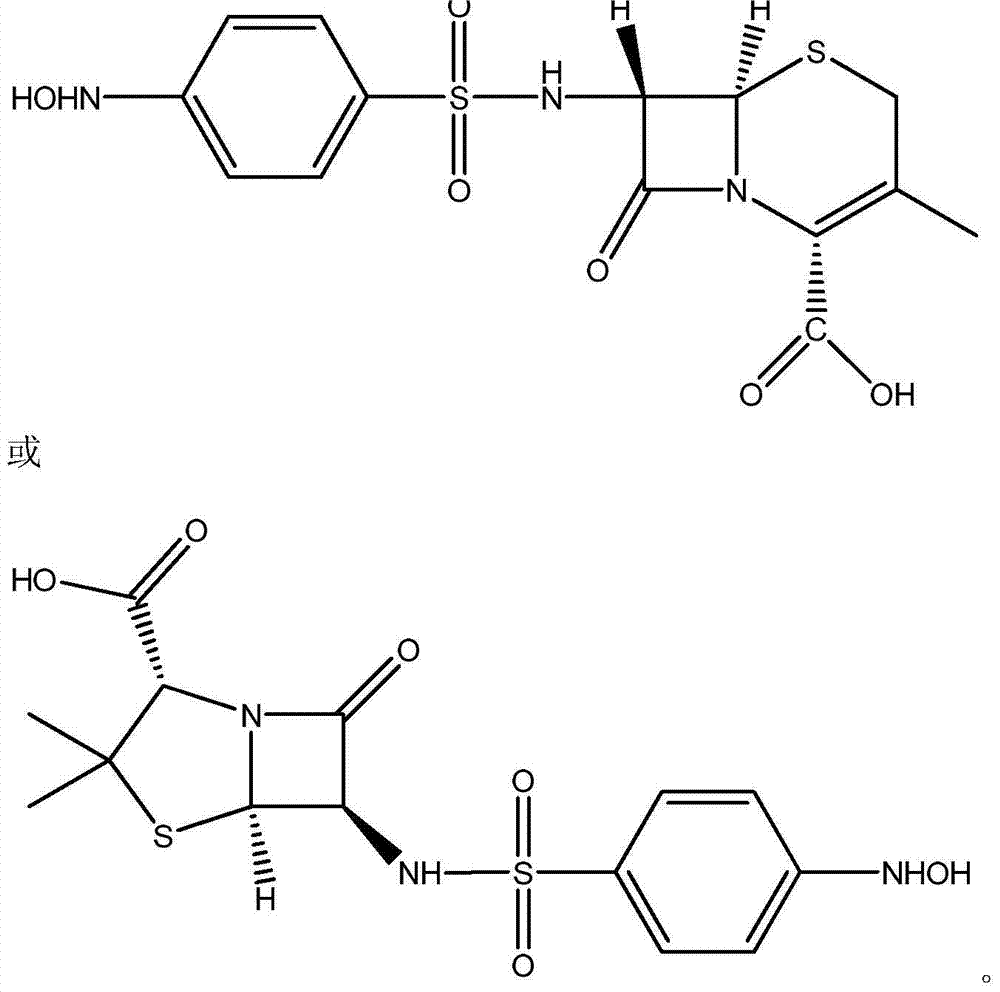
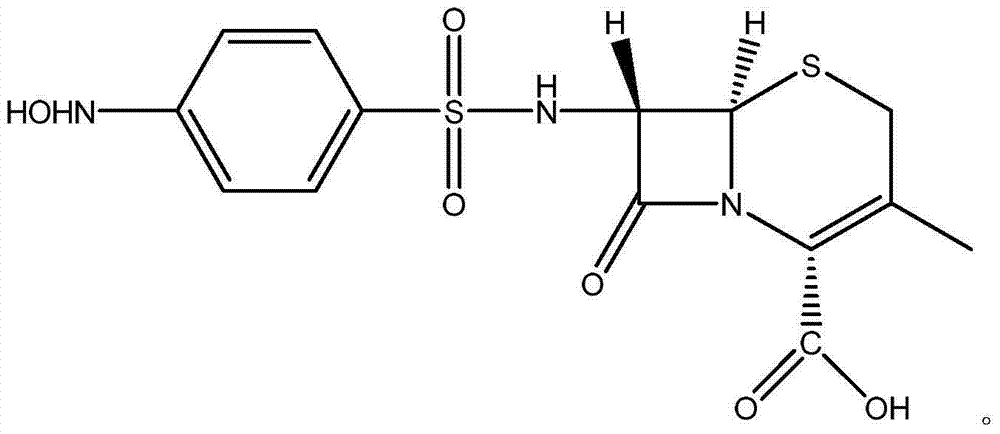
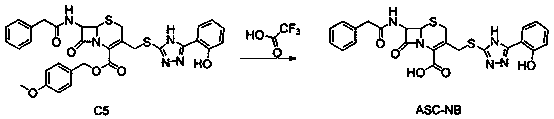Patents
Literature
34 results about "Beta lactams" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beta-lactamase inhibitors
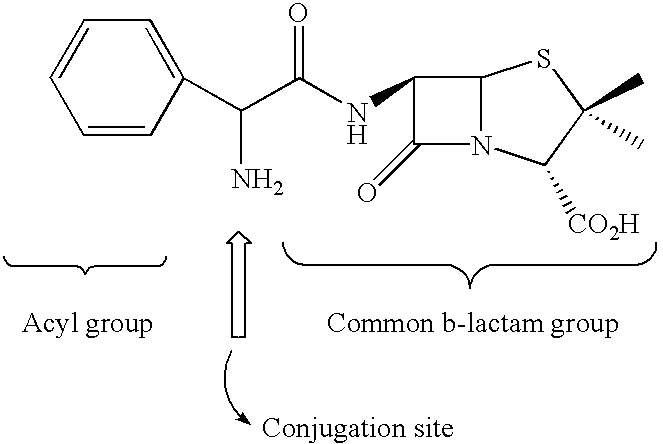
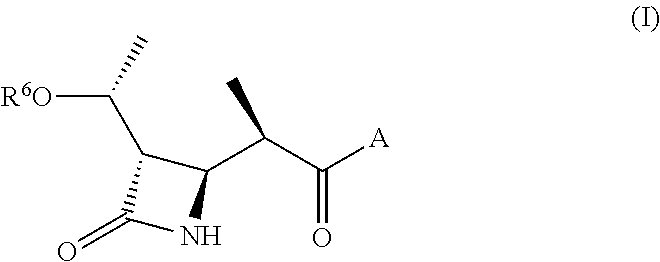
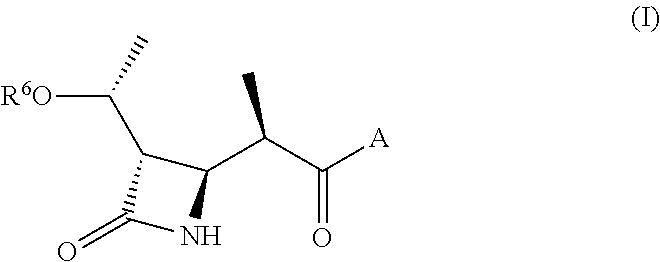
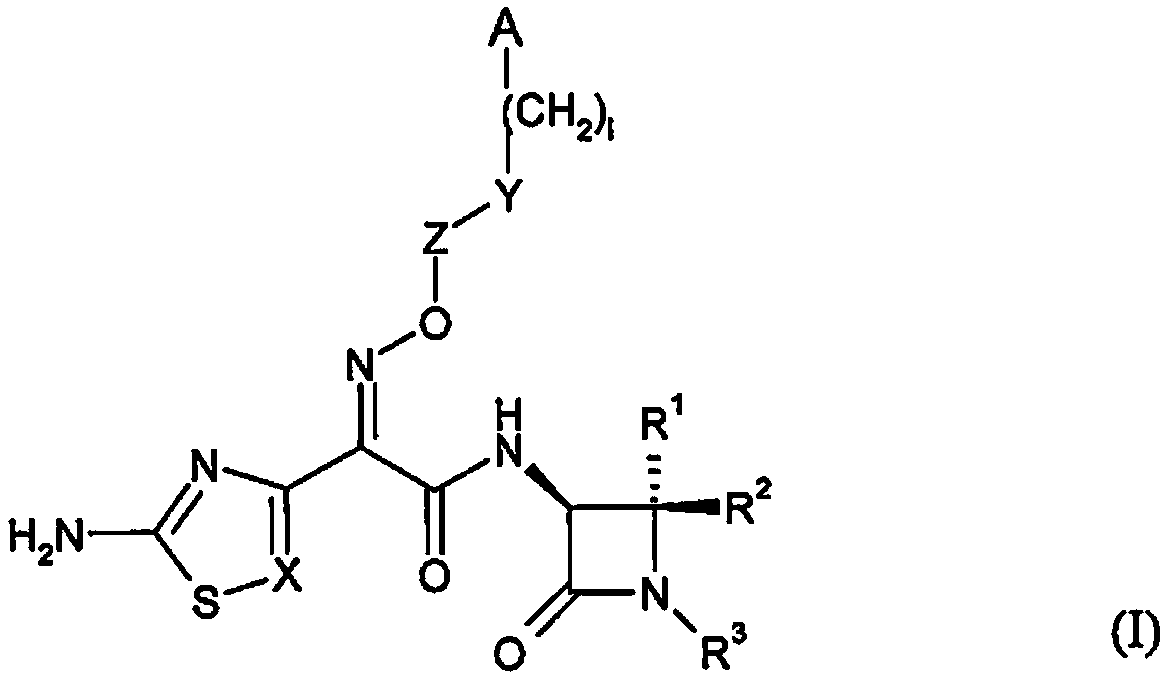
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Method for simultaneously detecting multi-kind pesticide residues in bee products
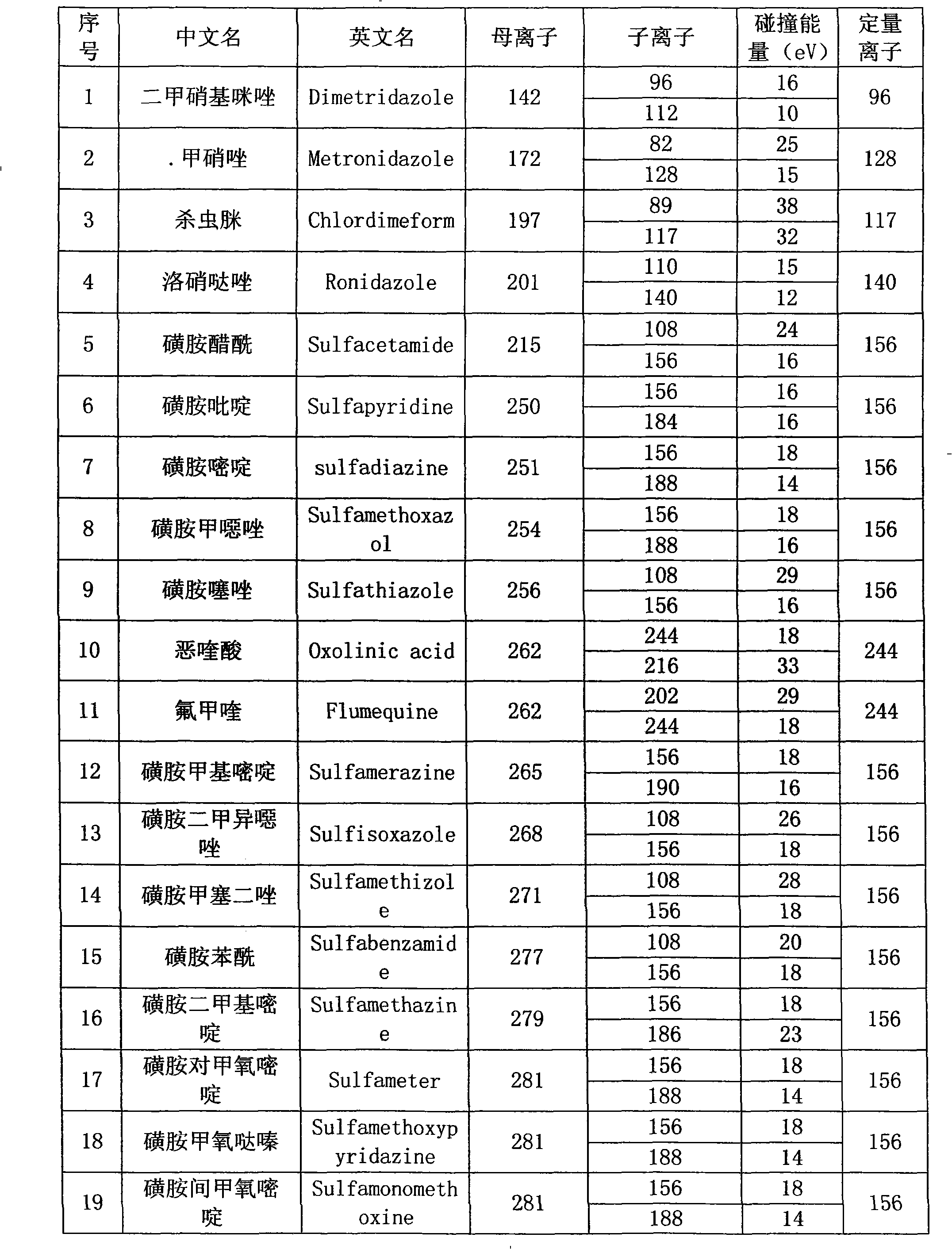
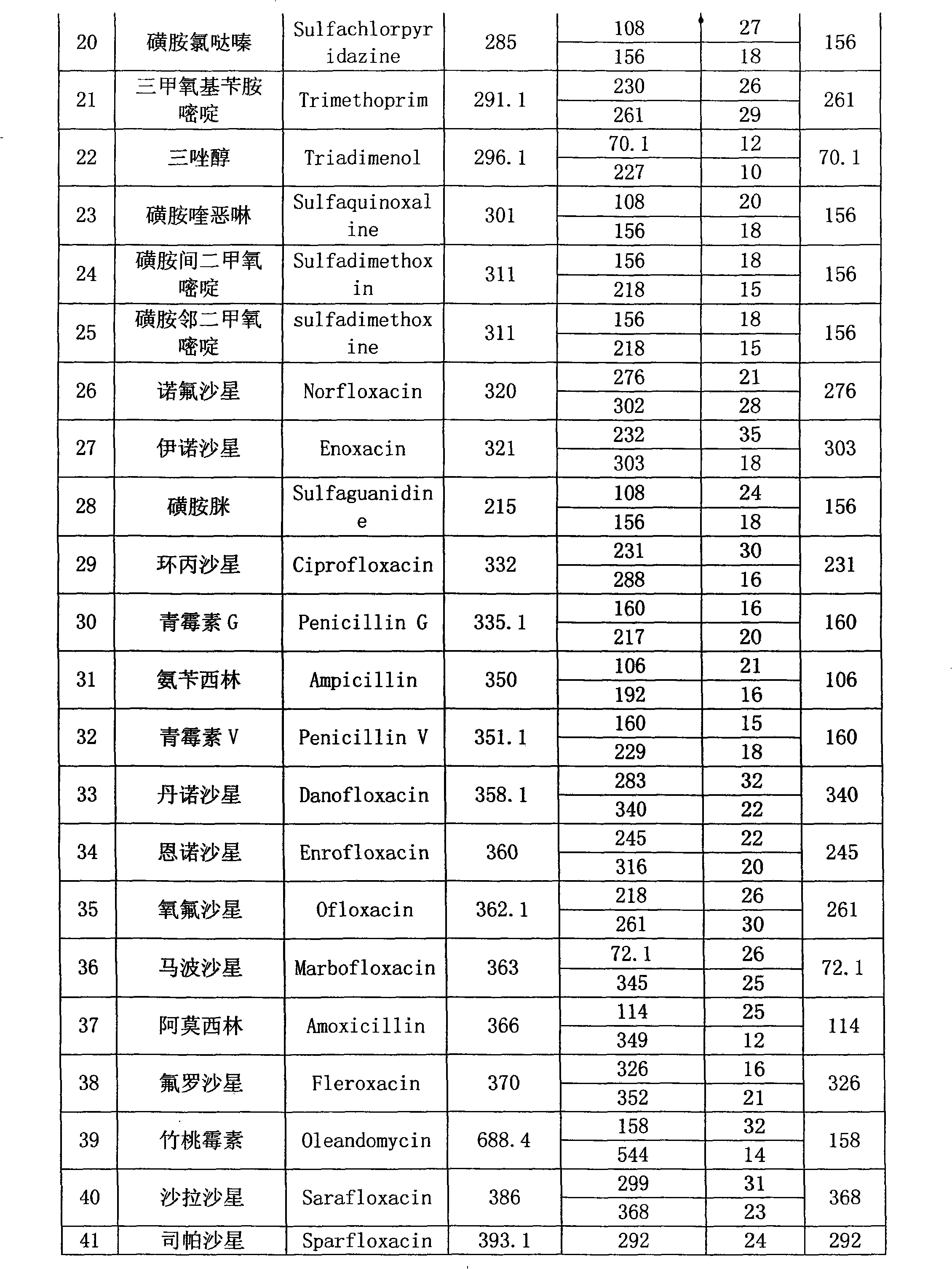
InactiveCN101358953ASolve the problem of matrix effectFast wayComponent separationRetention timePhosphate
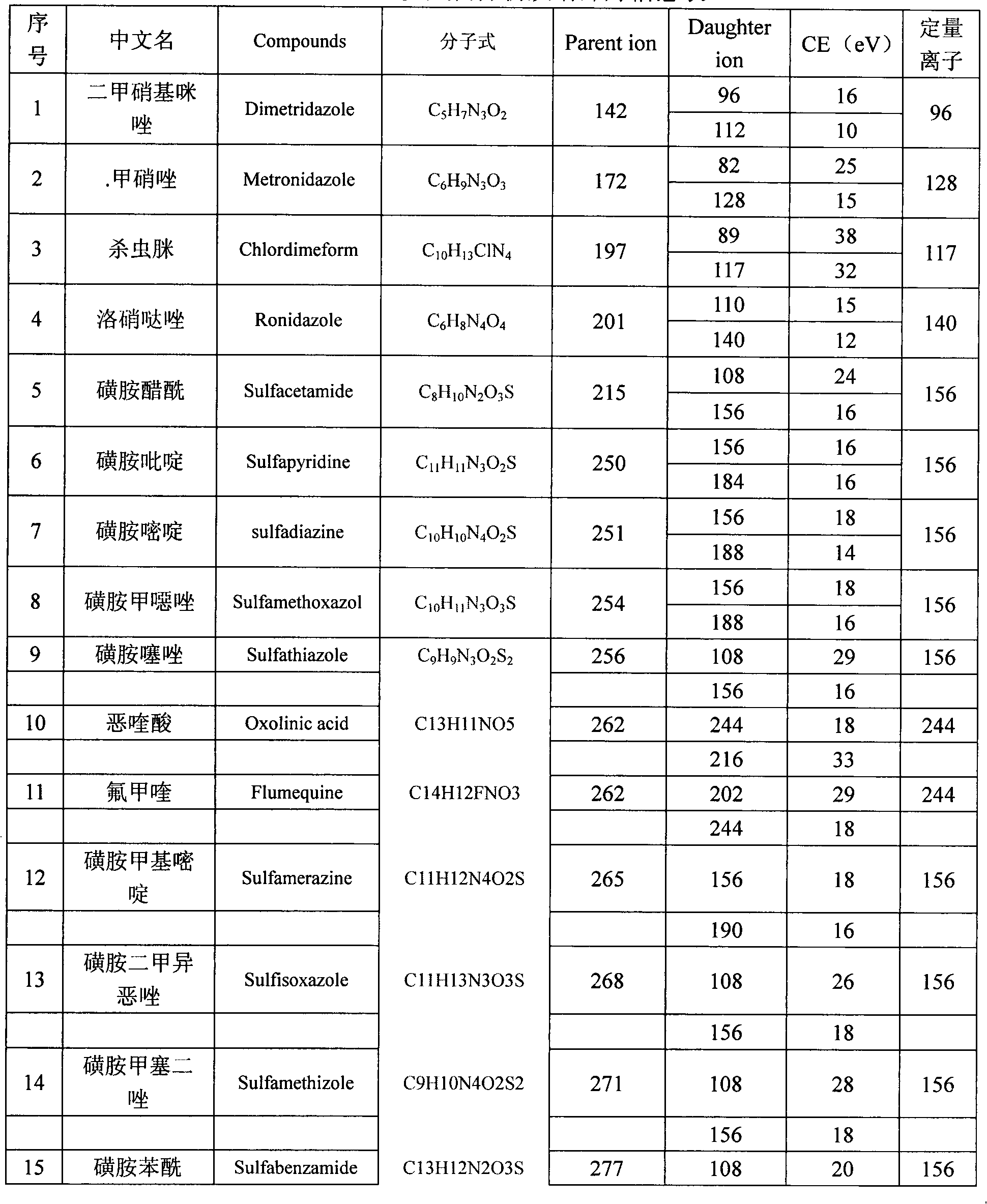
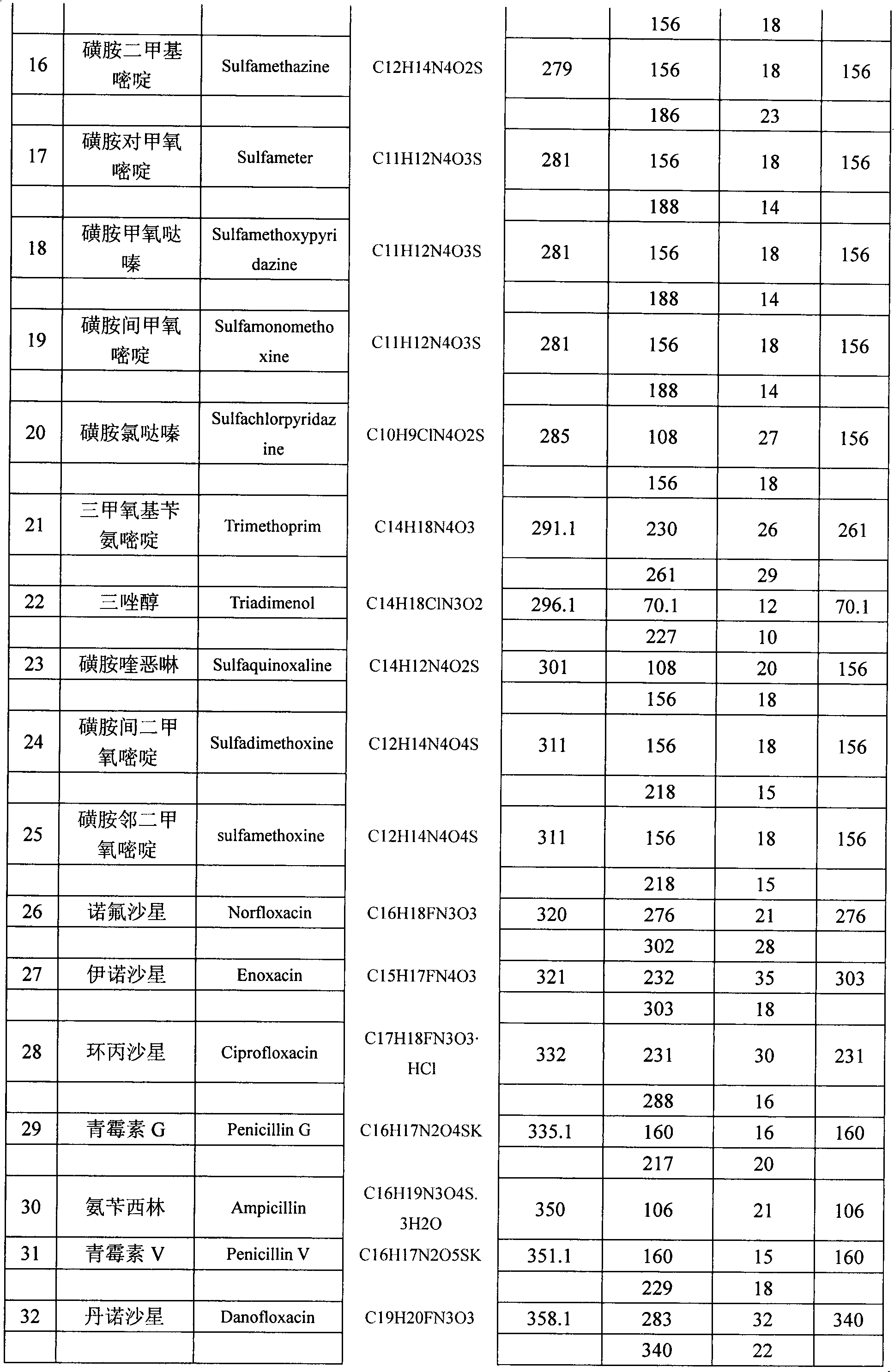
The present invention relates to a method of simultaneously detecting a plurality of agro-veterinary drug residues in bee products. The extracted liquid trichloroacetic acid or perchloric acid and the extracted liquid acetate, phosphate or borate solution are added into a sample; the pH value is controlled between 4.5 and 9.0; the mixed solution is centrifuged, the filtrate is added into a solid phase extraction column to be extracted, the extraction column is eluted and dried, the column is washed by oxalic acid-methanol solution, the volume of the eluent is defined by the aqueous solution of methanol, the eluent is added into liquid chromatography-tandem mass spectrometry to be analyzed and tested, the acquired chromatographic peak is contrasted with the known standard chromatographic peak of the drug, and according to the retention time and the abundance of the mass spectrum ions, the specific name of the detected drug is determined. The method only requires one pre-treatment of the sample, and thus can simultaneously extract 11 classes and more than 60 kinds of veterinary drug residues, such as sulfonamides, quinolones, macrolides, lincomycins, nitroimidazoles, beta-lactams, tetracyclines, chloromycetins, trinethoprims, chlordimeform, triadimenol and the like, the efficiency of analysis is high, and the detection cost is greatly reduced.
Owner:中华人民共和国江苏出入境检验检疫局
N-thiolated beta-lactams: novel antibacterial agents for methicillin-resistant Staphylococcus aureus
The invention relates generally to novel N-thiolated β-lactams. More specially, the invention relates to the use of these novel antibacterial agents in the treatment or inhibition of methicillin-resistant Staphylococcus aureaus.
Owner:SOUTH FLORIDA UNIVESITY OF
Β-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Detection method of residual quantities of various veterinary drugs in culturing or slaughtering environment
The invention provides a detection method of residual quantities of various veterinary drugs in a culturing or a slaughtering environment. With the method, rapid screening and quantitative detection can be carried out upon 61 drugs of 9 categories. The drugs include chlormycetin, beta lactams (penicillins), quinolones, sulfonamides, trimethoprims, macrolides, tetracyclines, and nitroimidazoles. According to the invention, a soil sample or an environmental water sample is added into a phosphate buffering solution; a filtrate obtained through centrifugation is extracted in a solid phase extraction column, and is eluted; the extraction column is dried by blowing, and is washed by using a methanol solution; an obtained eluent is titrated by using a methanol solution; a chromatogram peak of the sample is detected by using liquid chromatography-tandem mass spectrometry; the chromatogram peak is compared with a standard chromatogram peak, such that a specific variety of the detected drug is accurately determined. According to the invention, the sample solution is subject to liquid chromatography-tandem mass spectrometry multi-reaction monitoring selected ion analysis. Through internal standard correction, the recovering rate is 70-120%, and a relative standard deviation RSD is no larger than 18%. Compared with existing technologies, the analysis efficiency is improved by at least 5 times, and the detection cost is 30% of that of existing technologies.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
N-thiolated beta-lactams: novel antibacterial agents for methicillin-resistant staphylococcus aureus
The invention relates generally to novel N-thiolated beta-lactams. More specifically, the invention relates to the use of these novel antibacterial agents in the treatment or inhibition of methicillin-resistant Staphylococcus aureus.
Owner:SOUTH FLORIDA UNIVESITY OF
Method and kit for detecting, or determining the quantity of, beta-lactam penicillins
ActiveUS20030143653A1Biological material analysisPeptide preparation methodsBeta lactam antibioticLabelling
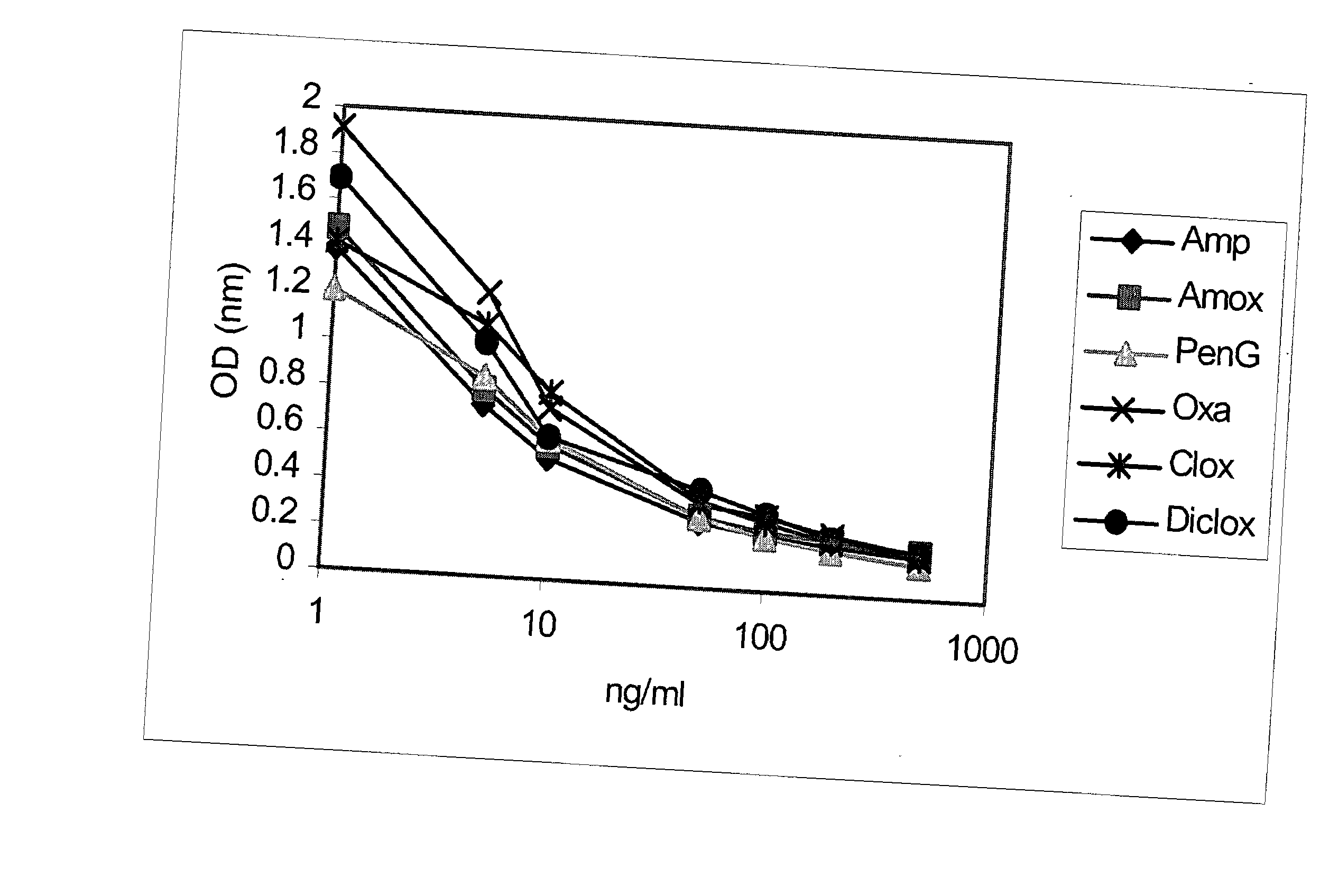
The invention provides a hapten comprising a 6-[D-alpha-aminoacetamido]penicillin derivative crosslinked at the alpha-amino group with a substituted or unsubstituted phenyldicarbaldehyde. In addition, the invention provides an immunogen comprising the aforementioned hapten coupled to an antigenicity-conferring carrier material, a conjugate comprising the aforementioned hapten coupled to a labelling agent, as well as, antibodies raised against the aforementioned immunogen and capable of binding with at least one structural epitope of an intact beta-lactam ring. The invention further provides a method and a kit for detecting, or determining the quantity of, beta-lactam antibiotics, as well as, use of the aforementioned conjugate with the aforementioned antibodies for detecting, or determining the quantity of, beta-lactam antibiotics. The present invention has broad specificity across the main first generation beta-lactams and can be used to test milk and meat and the like for the presence of residual beta-lactam antibiotics.
Owner:NORTHERN BANK LTD
Method for preparing compound beta lactam sodium salt/sodium benemid for injection
InactiveCN1698896ASolve the problem of insolubleAntibacterial agentsOrganic active ingredientsSodium saltBeta lactams
Disclosed is a method for preparing compound beta lactam sodium salt / sodium benemid for injection, which comprises dissolving 1-10 weight parts of probecid into 1-100 parts of water, charging sodium ion-containing alkaline solution, adjusting pH to 6-11 for reaction into salts, filtering and degerming through microporous filtering film, asepsis drying, or filtering with microporous filtering film and charging 10-100 parts of absolute ethyl alcohol or waterless acetone for evolution of solid body, carrying out asepsis dehydration obtain the end product, mixing sodium salt of beta-lactams with the obtained sodium benemid by the proportion of 10:1-1:10 and dissolving into 1-100 parts of water.
Owner:XI AN JIAOTONG UNIV
Immobilized alpha-amino-acid ester hydrolase, preparation and application thereof
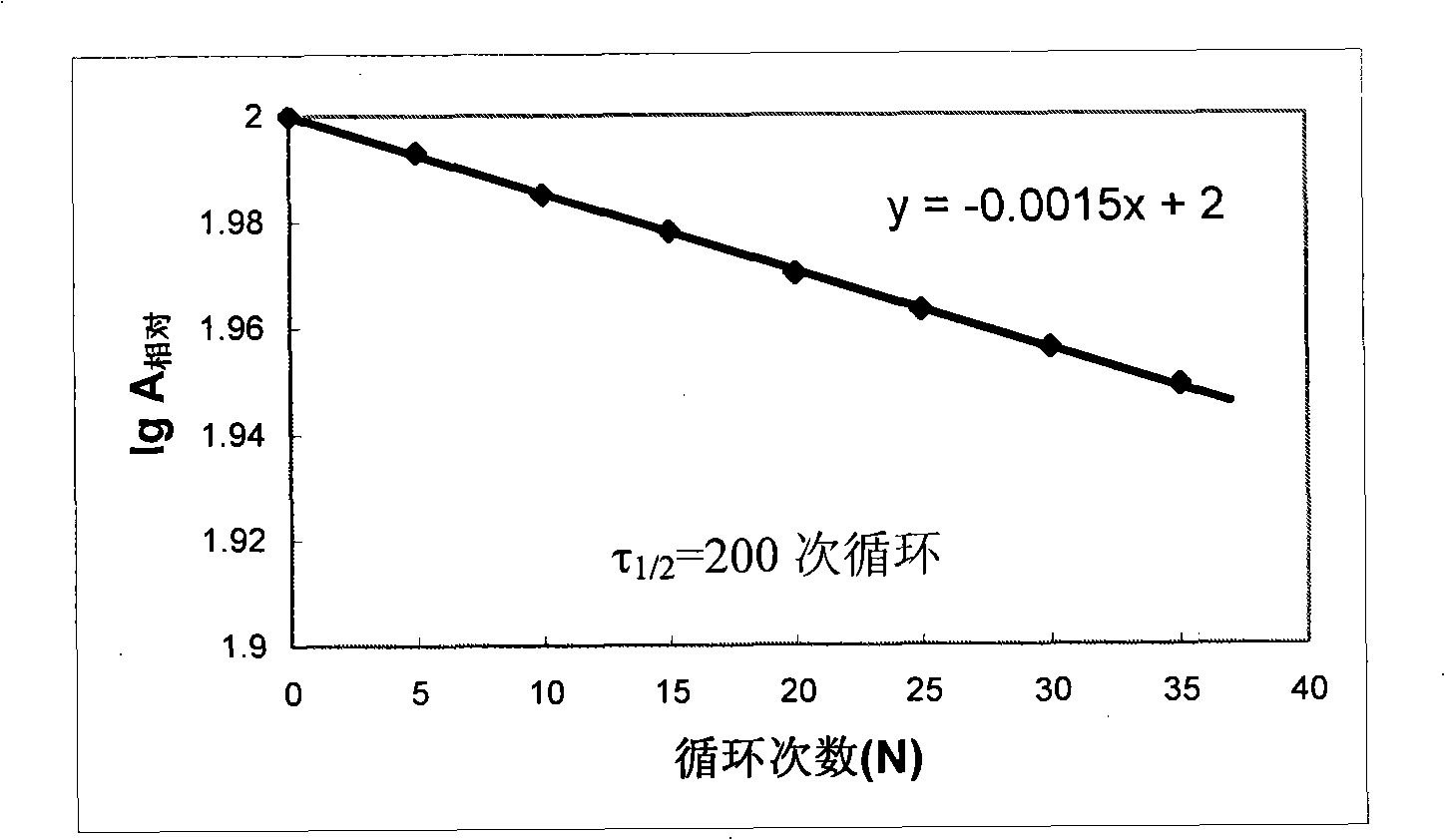
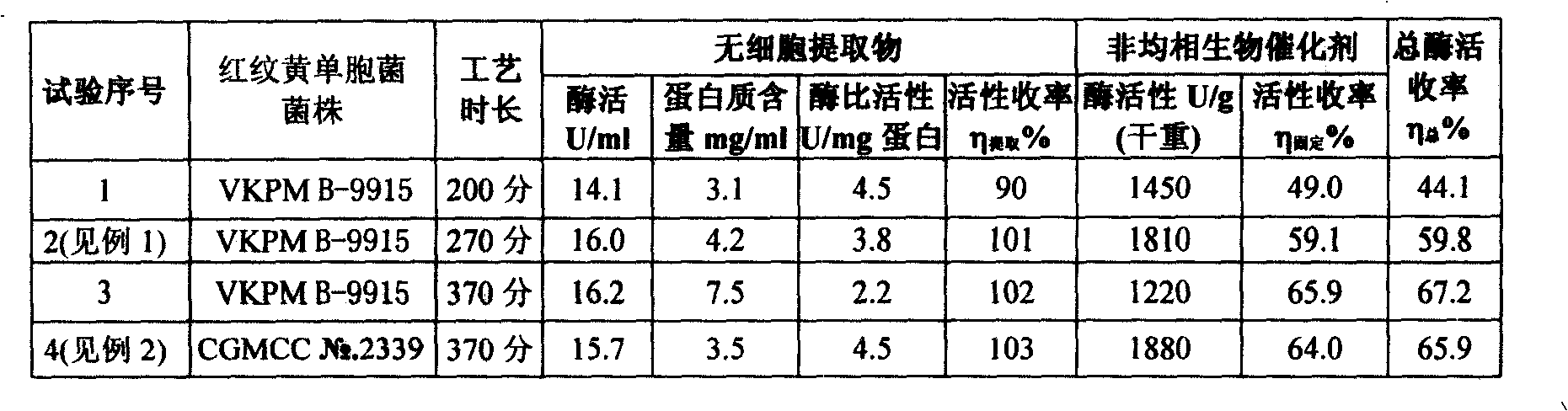
InactiveCN101525603AMany preparation stepsShort manufacturing cycleHydrolasesChemical industryCross-linkPenicillin
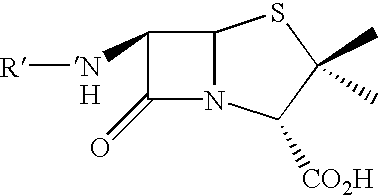
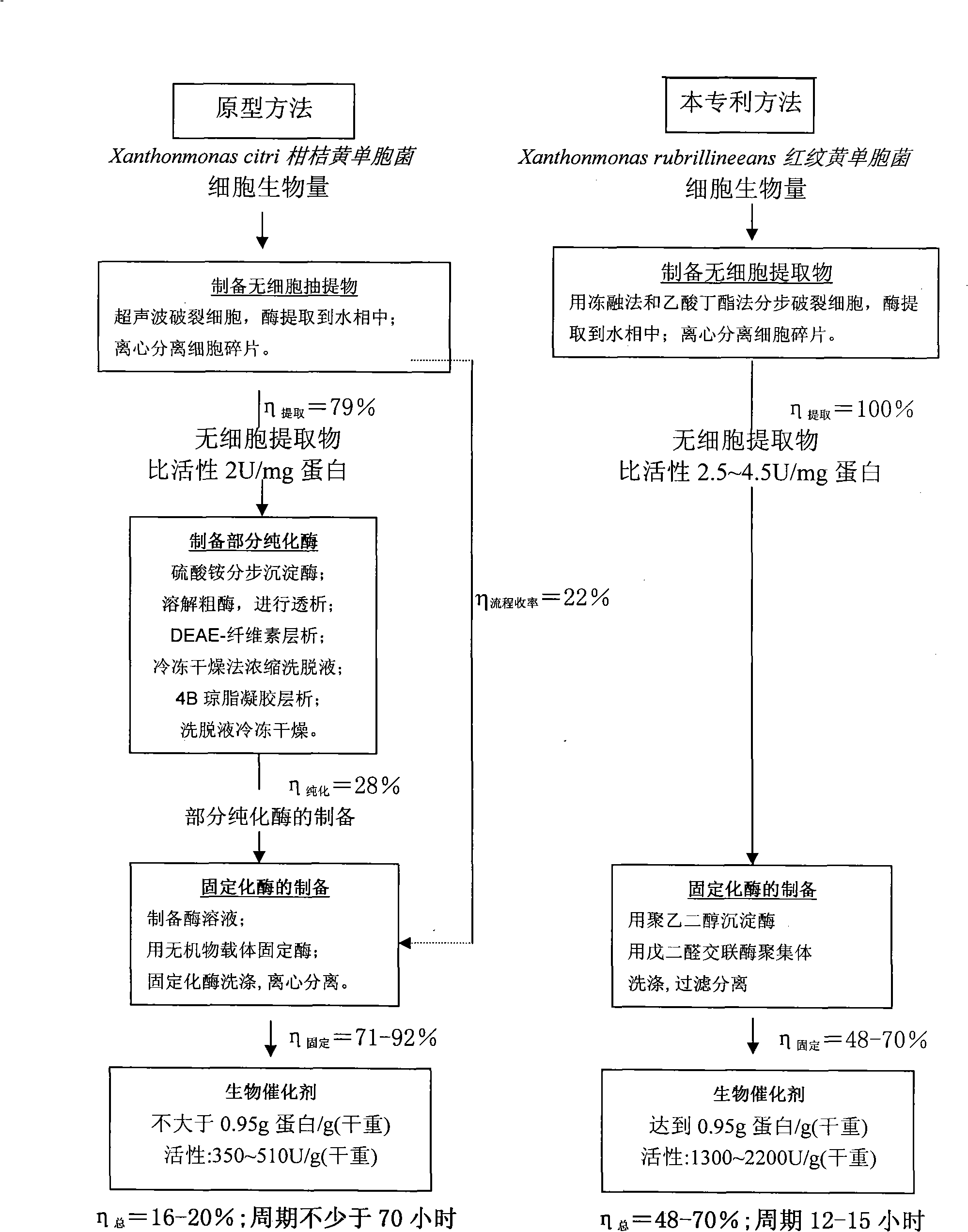
The invention discloses a heterogeneous biocatalyst taking alpha-amino-acid ester hydrolase as basis, a preparation method thereof and application in synthesizing amino beta-lactams. The activity of catalyst synthetase is 2,200 U / g by dry weight, and the content of protein is 0.95 g / g by dry weight; and xanthomonas rubrilineans cell biomass is processed in an organic solvent under a pH gradient through low temperature effect to extract enzyme, and enzyme aggregates are deposited and cross-linked to obtain the hydrolase. The alpha-amino-acid ester hydrolase as a catalyst can be synthesized into amino penicillin and amino cephalosporin drugs through acylating a beta-lactam compound by D-phenylglycine methyl ester derivatives in water or in a mixed medium of water and an organic solvent.
Owner:SICHUAN INDAL INST OF ANTIBIOTICS CHINA NAT PHARMA GROUP CORP +1
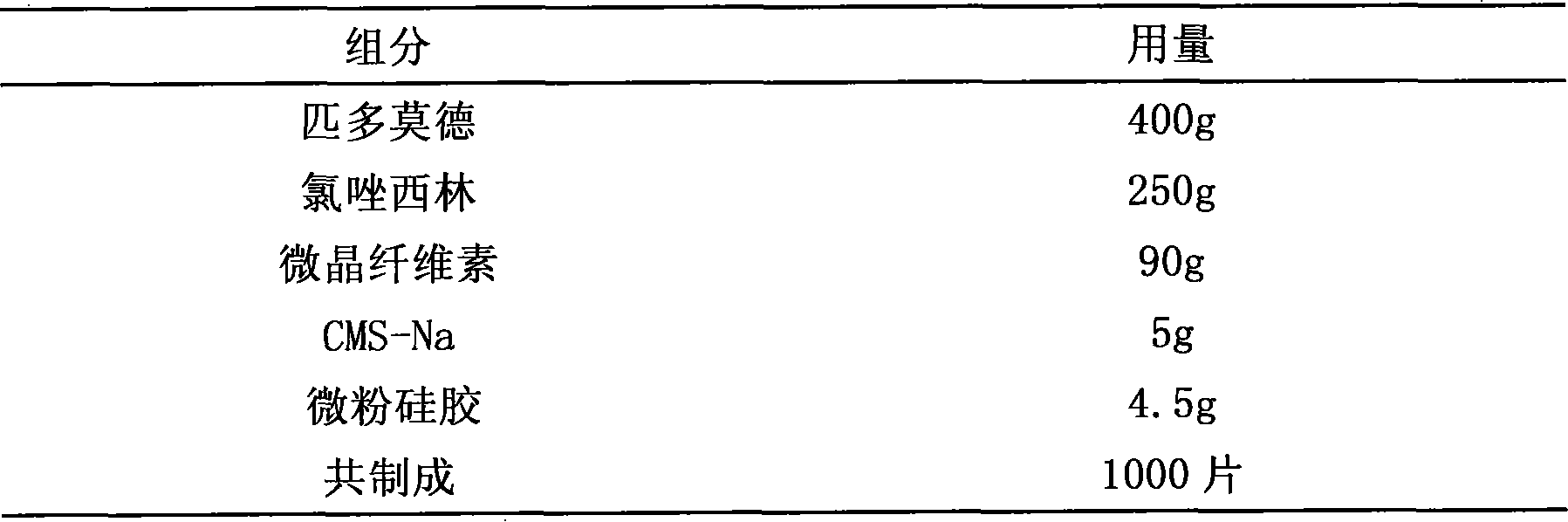
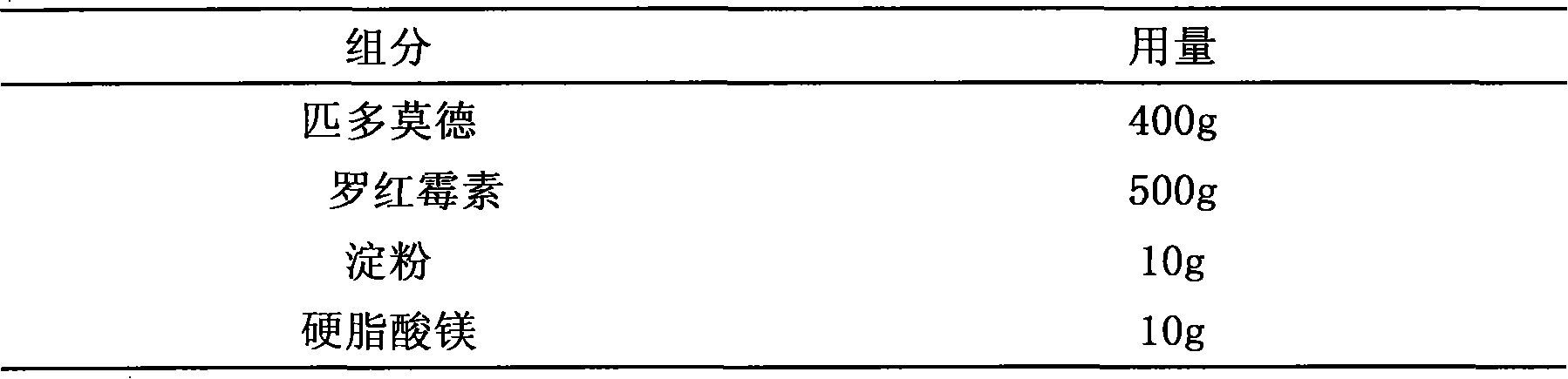
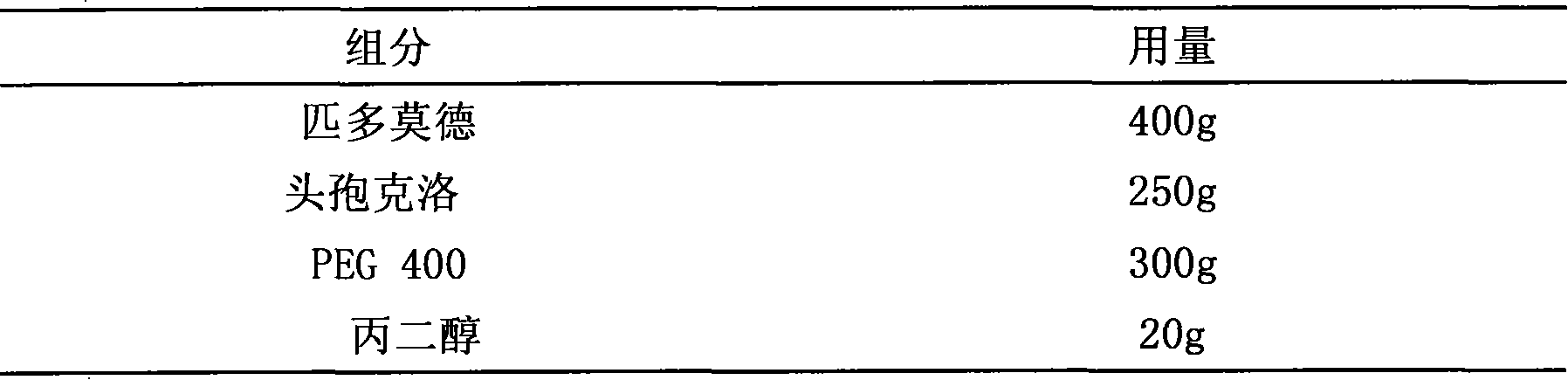
Pidotimod-containing pharmaceutical composition and preparation method thereof
InactiveCN101612152AAntibacterial agentsHeterocyclic compound active ingredientsAntifungalPenicillin
The invention provides a pidotimod-containing pharmaceutical composition and a preparation method thereof. The pidotimod-containing pharmaceutical composition consists of pidotimod and any antibacterial medicament. The antibacterial medicament may be any of beta-lactams (including as penicillins, cephems and unclassical beta-lactams), aminoglycosides, polymyxins, macrolides, lincomycins, norvancomycins, tetracyclines, chloromycetins, artificial antibacterial medicaments (including sulfonamides, quinolones and furofurans), cholesterols and antiviral medicaments. The unit dosage of the pidotimod is 0.1 to 2 grams, preferably 0.4 to 0.8 gram. The dosage of the antibacterial medicament is determined according to the dosage range of the antibacterial medicament. The composition of the pidotimod and the antibacterial medicament can exogenously and directly kill pathogenic bacteria through the antibacterial medicament and mobilize endogenous anti-infection immunity through an immunomodulator to improve anti-inflection effect, and has excellent synergy.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
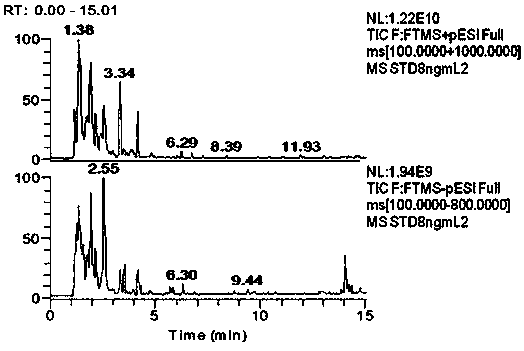
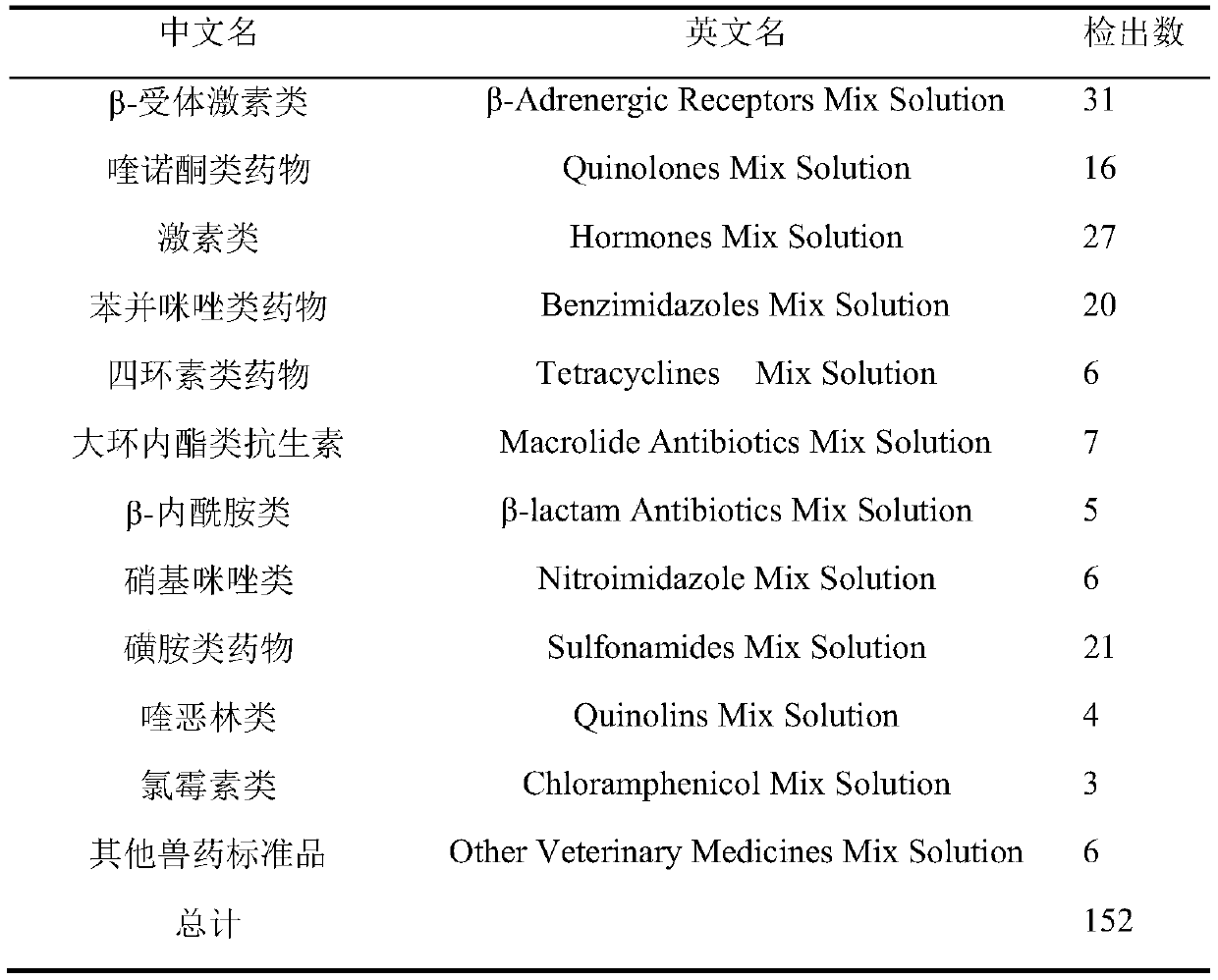
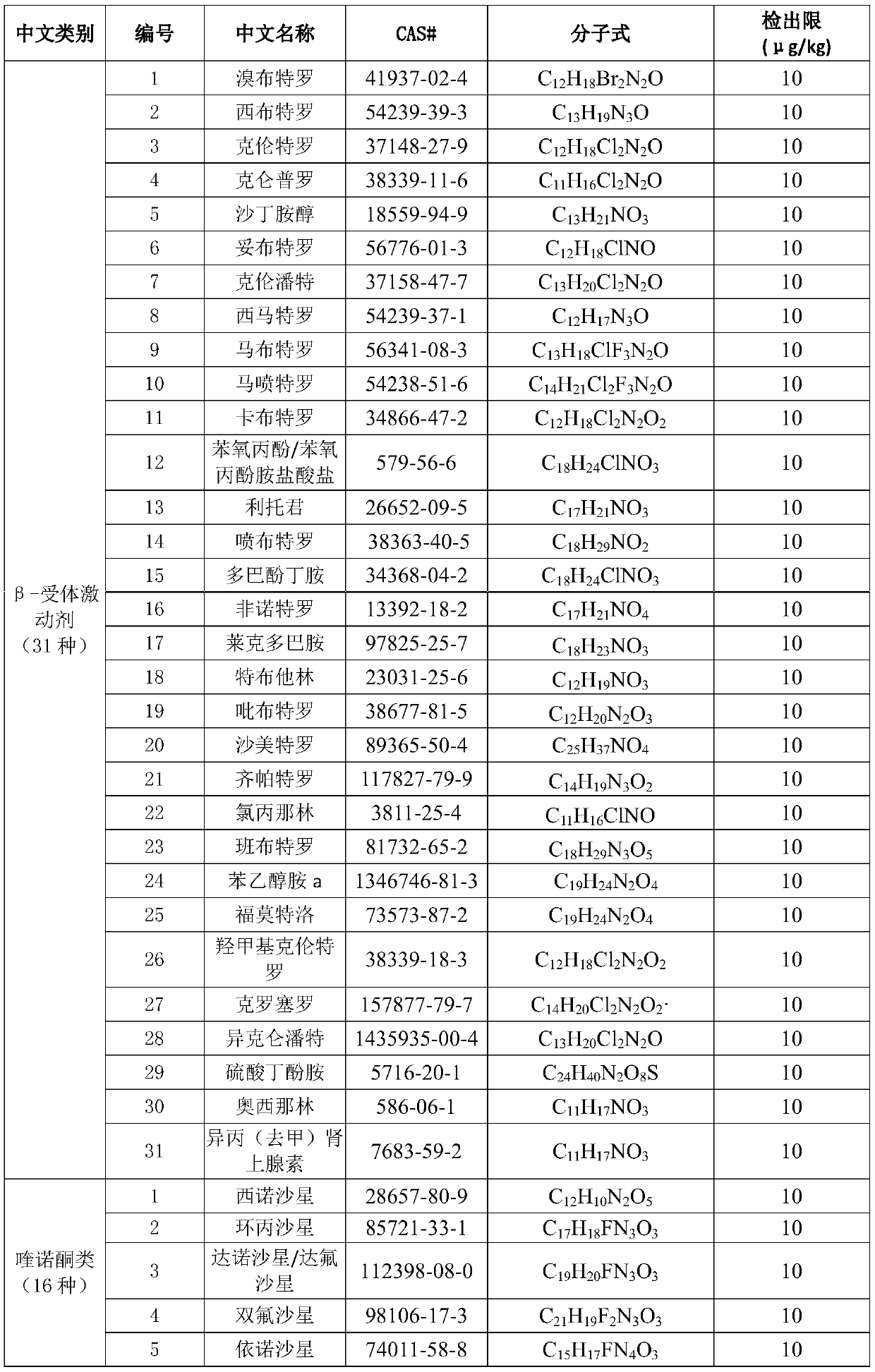
High-resolution mass spectrometry method for synchronously detecting 152 chemical pollutants in livestock and poultry meat
InactiveCN110530991ASignificant technological progressHigh detection throughputComponent separationQuinoxalineNitroimidazole
The invention provides a high-resolution mass spectrometry method for synchronously detecting 152 chemical pollutants in livestock and poultry meat. The method comprises a step of establishing a chemical pollutant standard quality spectrum library, a sample detection step and a screening analysis step. The method covers 152 target compounds such as sulfanilamides, quinolones, quinoxalines, nitroimidazoles, beta-lactams, tetracyclines, macrolides, chloramphenicols, benzimidazoles, nicotinic agonists, beta-receptor agonists, hormones and the like. The method has the advantages that the sample pretreatment method is simple and convenient; the 152 compounds can be screened and confirmed by one-needle sample injection; the detection flux is large; the accuracy is high; and the method is suitable for simultaneous qualitative confirmation detection of various veterinary drug residues in the livestock and poultry meat.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
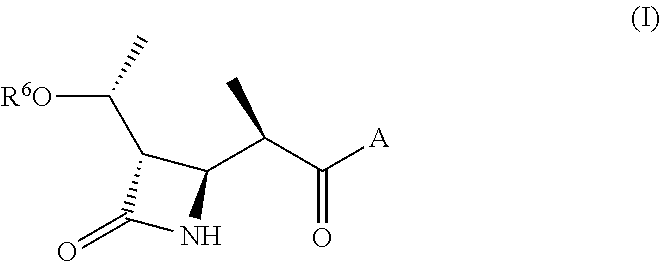
Substituted beta-lactams
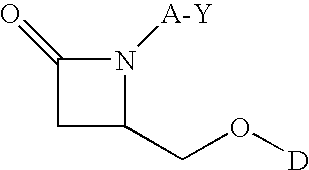
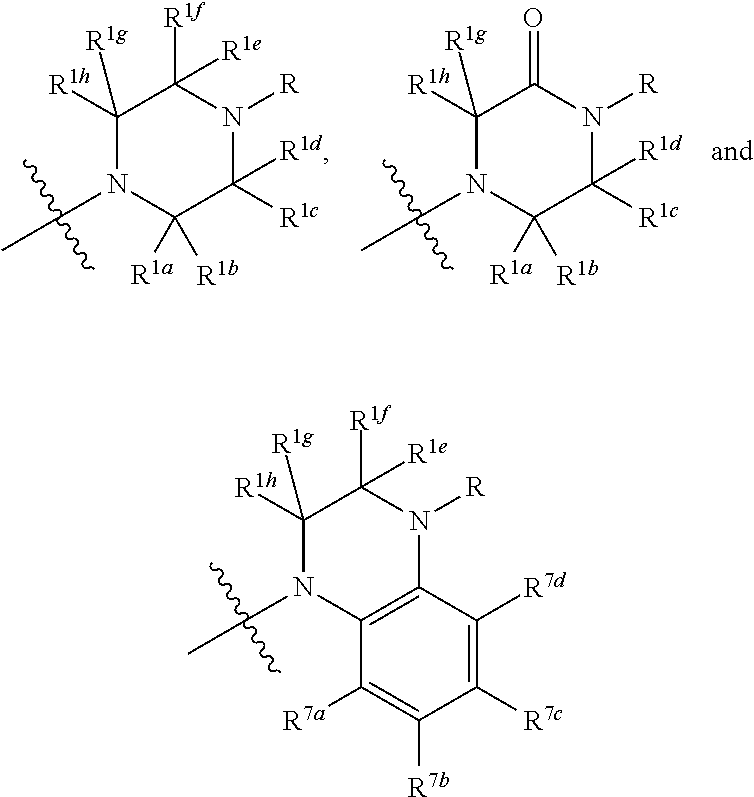
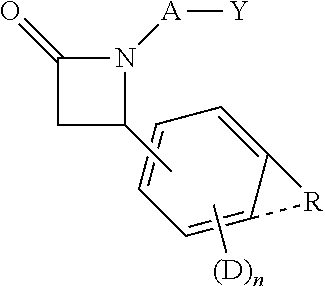
Disclosed herein is a compound comprising a formulaor a pharmaceutically acceptable salt or a prodrug or a metabolite thereof;Y isA is —(CH2)6—, cis —CH2CH═CH—(CH2)3—, or —CH2C≡C—(CH2)3—, wherein 1 or 2 carbon atoms may be substituted with S or O; or A is —(CH2)m—Ar—(CH2)o— wherein Ar is interarylene or heterointerarylene, the sum of m and o is from 1 to 4, and wherein one CH2 may be substituted with S or O; andD is aryl or heteroaryl.Methods of use are also disclosed.
Owner:ALLERGAN INC
Antibiotic Combinations For Providing Total Solution to the Treatment of Infections
InactiveUS20090318378A1Improve efficacyImprove securityAntibacterial agentsBiocideHospitalized patientsNosocomial pneumonias
The invention relates to a new pharmaceutical composition, a method of treatment of infection and also a process to prepare the composition. The infectious complications are important causes of morbidity and mortality. Hospital acquired pneumonia (HAP) remains the most severe nosocomial infection in intensive care units. Beta-lactams alone are always considered inadequate when P. aeruginosa and / or methicillin-resistant S. aureus are implicated as pathogens or copathognes. The present invention provides the desired empirical therapy for control of all bacterial infections. The invention provides antibiotic combination products for delivering at least two different antibiotics, through parenteral dosage form comprising protein-synthesis-inhibiting antibiotic which is amikacin or its sulphate salt and non-protein-synthesis-inhibiting antibiotic which is cefepime or its hydrochloride salt. The invention provides a total solution, against multiresistant P. aeruginosa, or Acinetobacter spp. and / or methicillin-resistant S. aureus, and are useful for intramuscular or intravenous administration as antibiotics for hospitalized patients with acute or serious infections. The pharmaceutical compositions described here normally have the least nephrotoxicity and have better efficacy and safety of cefepime plus amikacin combination.
Owner:VENUS REMEDIES LTD
Fluorescent indicator combination, fluorescence array sensor, preparation methods of fluorescent indicator combination and fluorescence array sensor and application
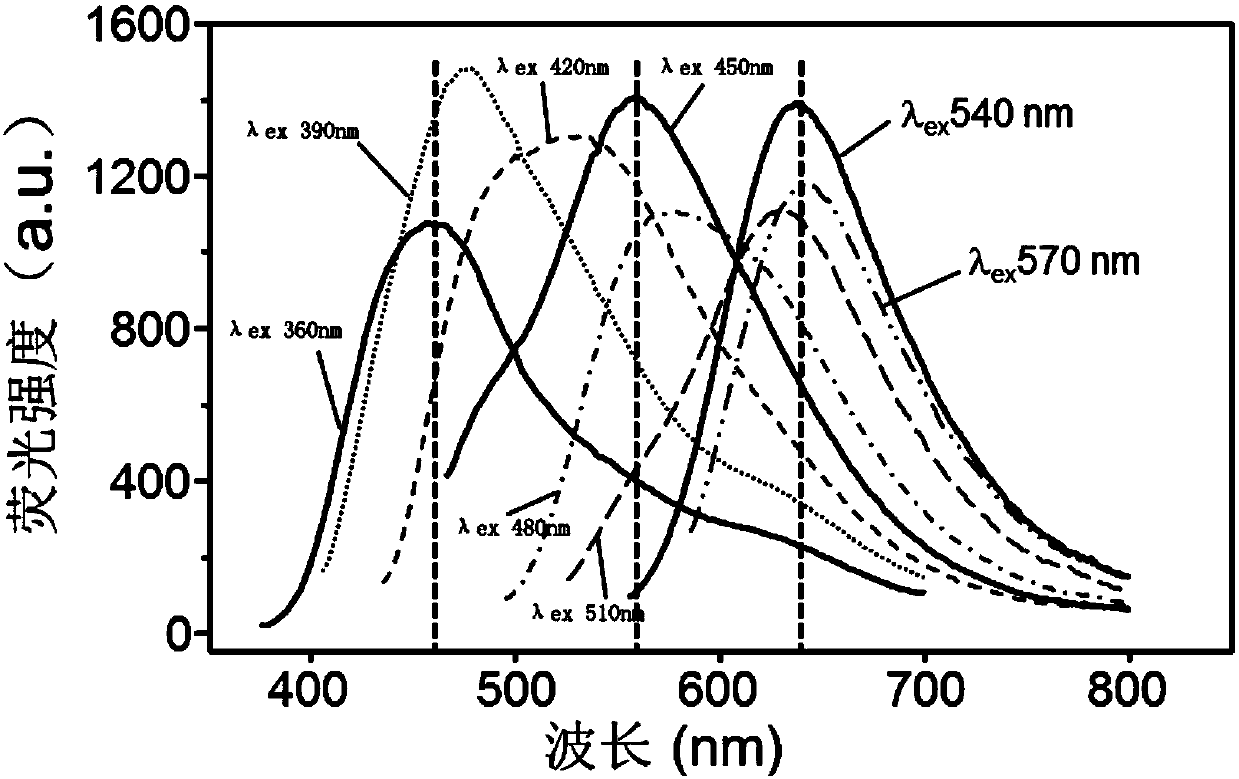
ActiveCN109975253AEasy to makeHigh detection sensitivityFluorescence/phosphorescenceAgainst vector-borne diseasesAntibiotic YQuantum
The invention discloses a fluorescent indicator combination, a fluorescence array sensor, preparation methods of the fluorescent indicator combination and the fluorescence array sensor and application. The fluorescent indicator combination comprises a plurality of carbon quantum dot-metal ion complexes formed by full-color fluorescent carbon quantum dots and various metal ions under conditions ofdifferent pH values. The fluorescent array sensor contains a fluorescent indicator; the sensor comprises a plurality of groups of response points; each group of response points comprise three or moreindependent response points respectively corresponding to excitation wavelengths of 360 nm, 450 nm, and 540 nm; and each of the response points comprises at least one carbon quantum dot-metal ion complex. The fluorescent array sensor of the invention can realize qualitative and semi-quantitative detection of four kinds of antibiotics such as tetracyclines, quinolones, beta-lactams and aminoglycosides; and the preparation process of the sensor is simple; the storage time of the sensor is long; the detection sensitivity of the sensor is high; and the sensor can realize simultaneous differentiation and detection of a plurality of antibiotics.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Treatment of chlamydiaceae infections by means of beta-lactams
InactiveUS20130053336A1Avoid problemsAvoid tissue damageAntibacterial agentsBiocideDiseaseChlamydiaceae Infections
The invention relates to a method for treating infections of bacteria of the Chlamydiaceae family using a β-lactam. The invention also relates to a method for treating diseases caused by an infection of bacteria of the Chlamydiaceae family using a β-lactam.
Owner:CENT NAT DE LA RECHERCHE SCI +1
Antibacterial drug for targeted therapy of staphylococcal infection by synergizing with antibiotic as well as synthesis method and application of antibacterial drug
InactiveCN108794508AIncrease vitalityExcellent synergistic antibacterial efficacyAntibacterial agentsOrganic active ingredientsAntibacterial agentTarget therapy
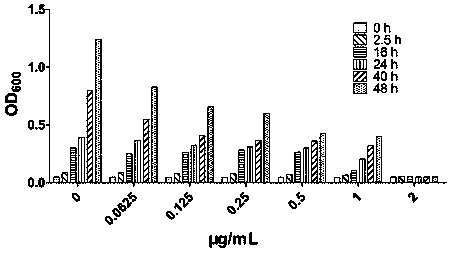
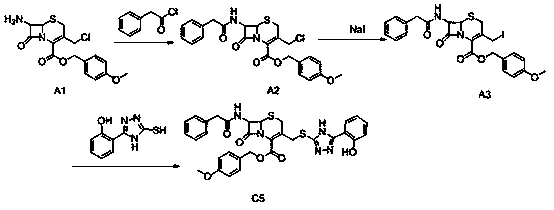
The invention designs and synthesizes a novel antibacterial drug ASC for staphylococcus aureus based on a beta-lactam ring of a parent nucleus structure of beta-lactam antibiotic molecules, wherein the ASC is an antibacterial reagent and is also a broad-spectrum inhibitor of beta-lactam antibiotic drug-resistant target protein metal beta-lactamase; the ASC can synergize with three kinds of 7 to 8antibiotics such as beta-lactams, aminoglycosides and tetracyclines to carry out targeted therapy on the staphylococcal infection. The vitality of the antibiotics is increased by 4 to 128 times by combining with 1 [mu]g / ml dosage of ASC.
Owner:NORTHWEST UNIV(CN)
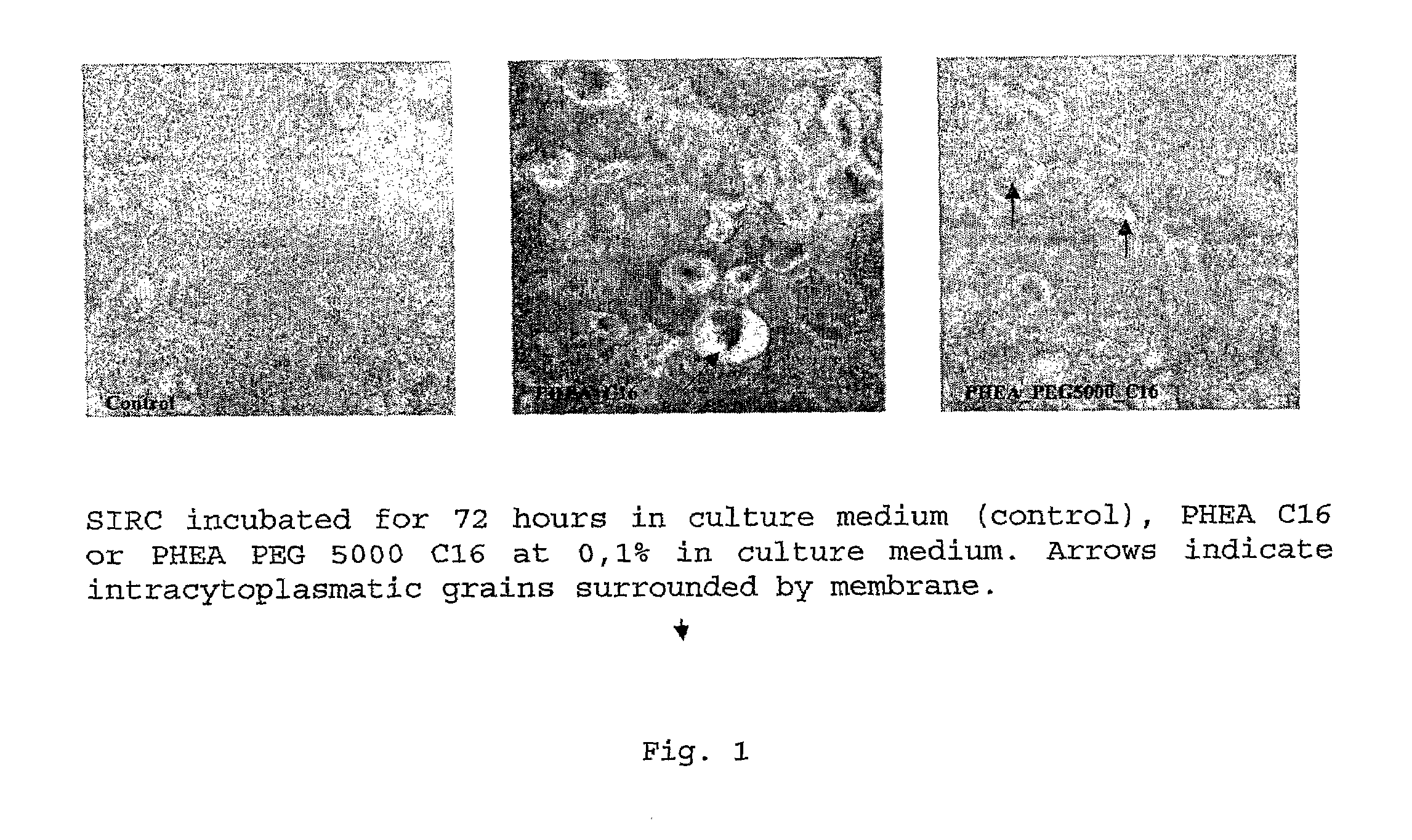
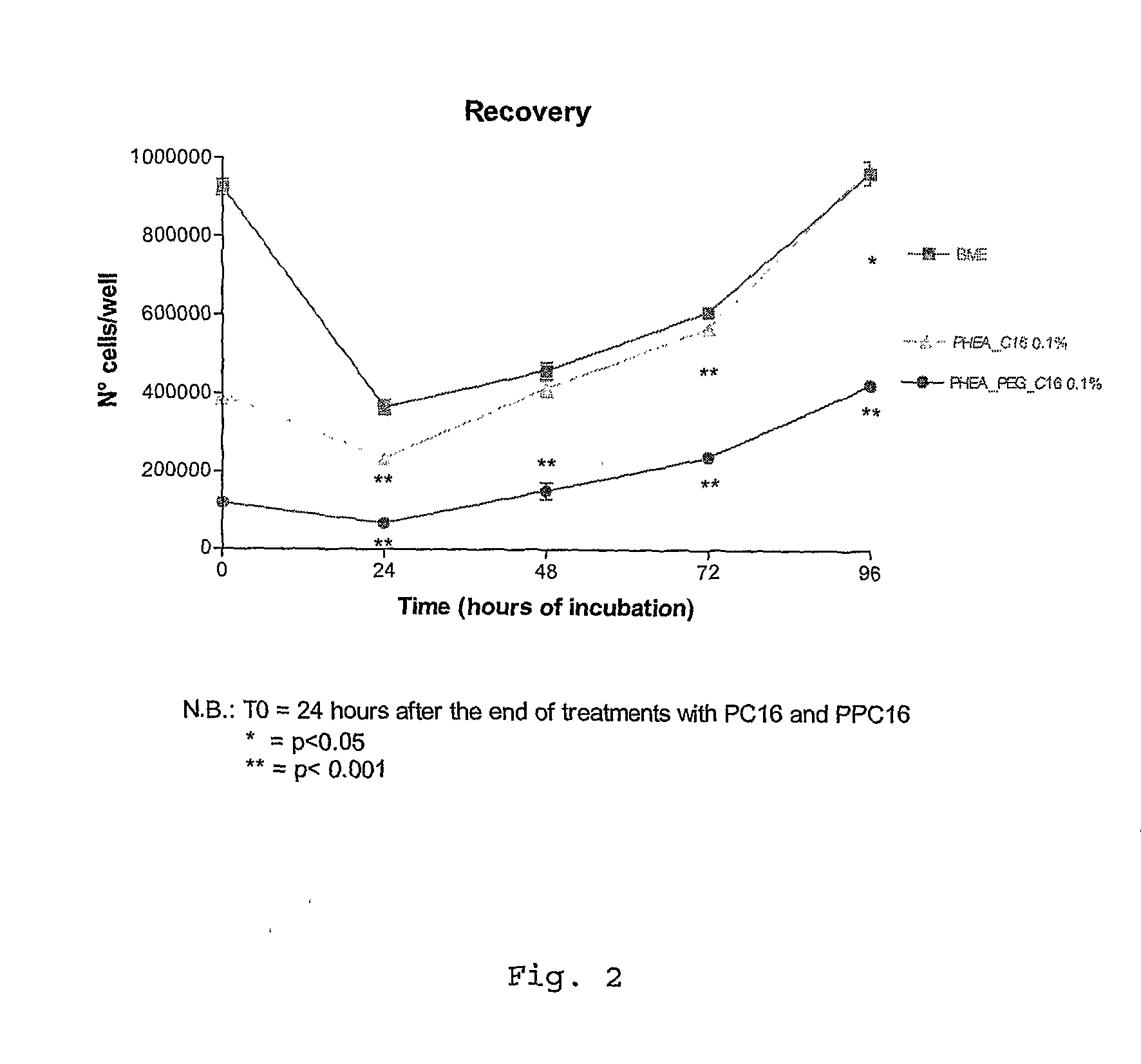
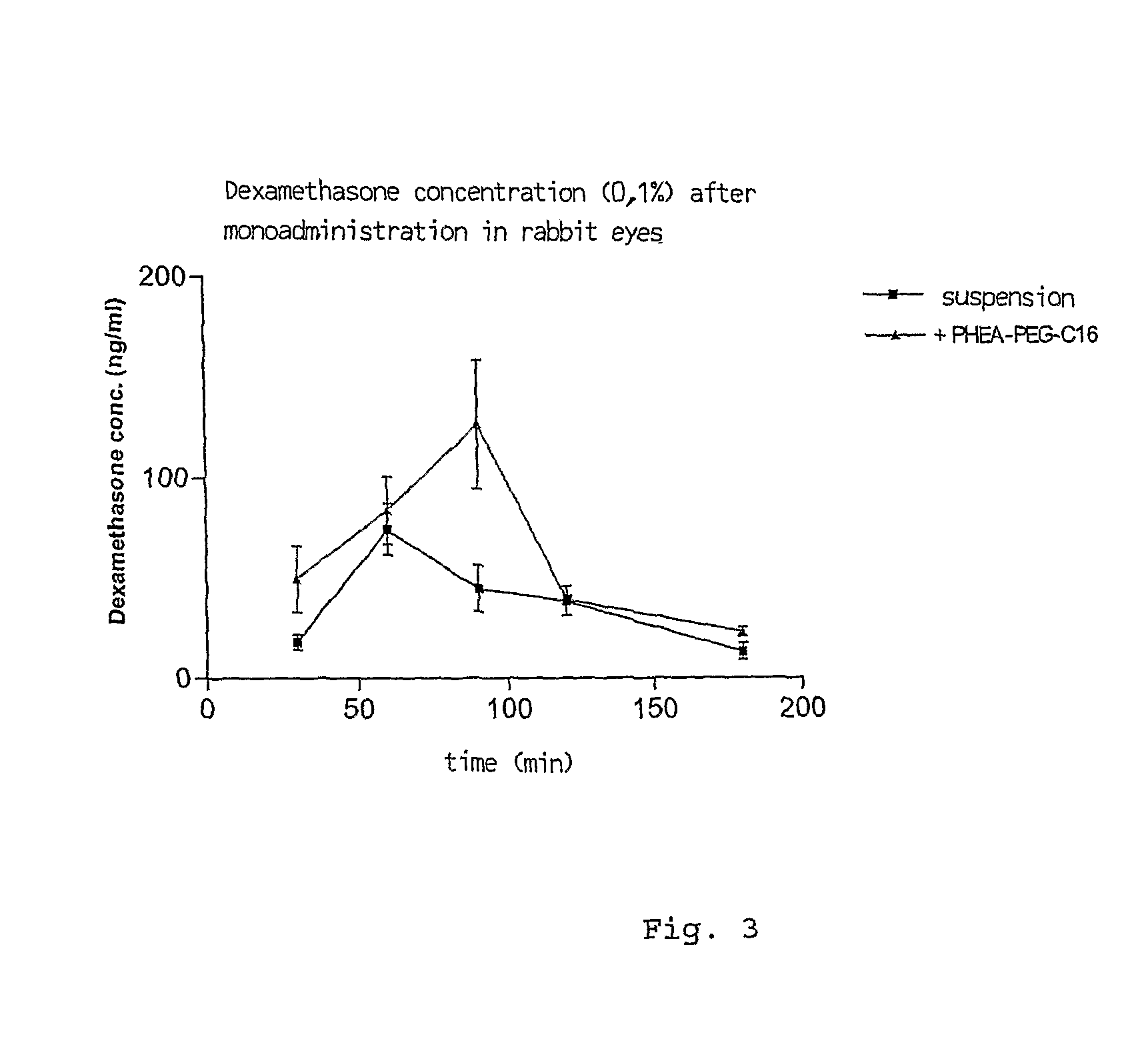
Ophthalmic Pharmaceutical Composition Containing Amphiphilic Polyaspartamide Copolymers
InactiveUS20090221545A1Increase the number ofLess non-productive absorptionBiocideAnimal repellantsPenicillinBeta blocker
The present invention relates in general to the use of amphiphilic graft-type copolymers of polyaspartamide for the ophthalmic administration of drugs, such as for example steroidal and non-steroidal anti-inflammatory agents, antimicrobial agents such as aminoglycosides, macrolides, cephalosporin, tetracycline, quinolones, penicillin, beta-lactams, anti-glaucoma agents such as prostaglandins, alpha- and beta-blockers, inhibitors of carbonic anhydrase, cannabinoids, antiviral agents, diagnostic agents, anti-angiogenic agents, antioxidants.
Owner:S I F I SPA
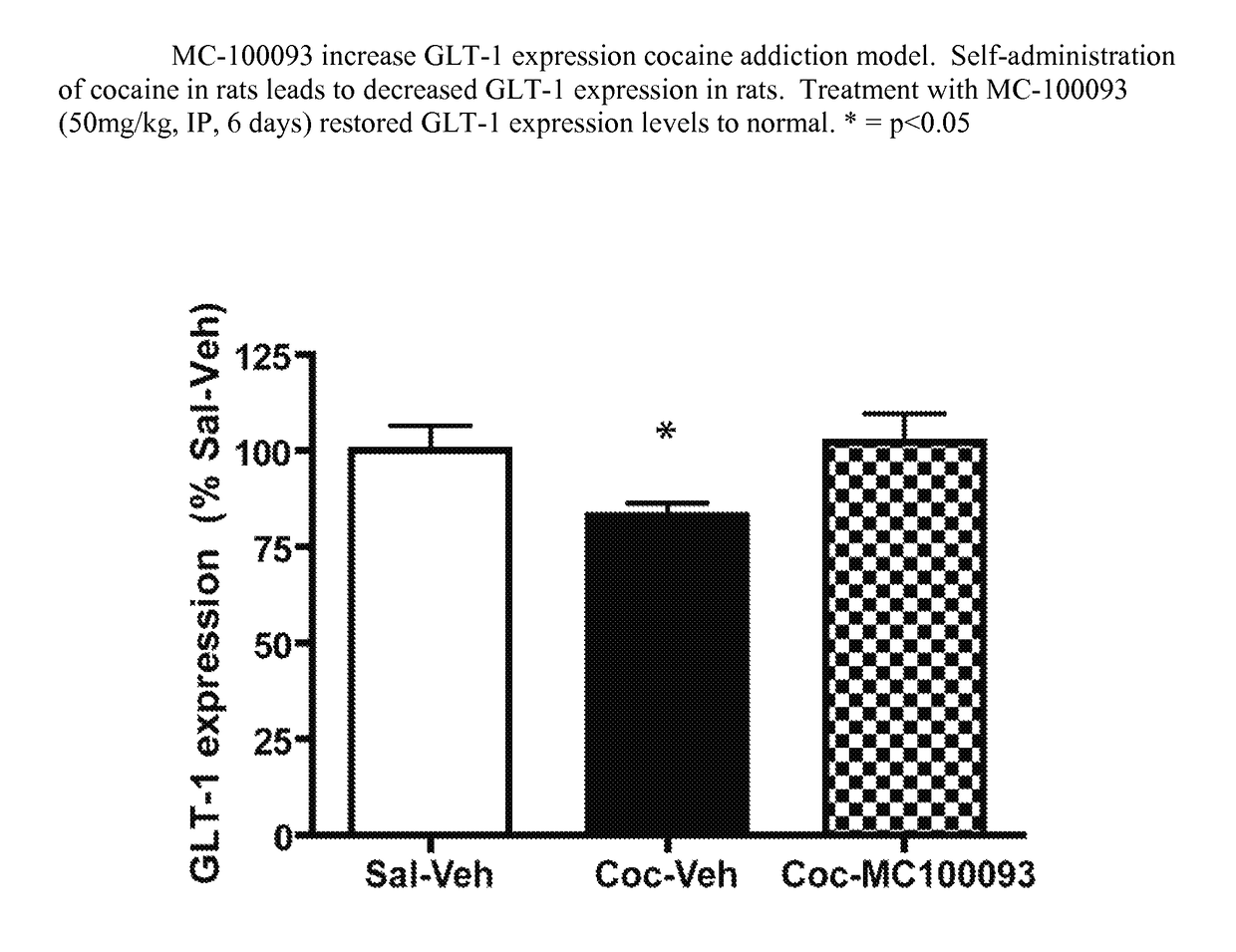
Beta lactams as modulators of glutamate uptake and methods for use thereof
Pharmaceutical compositions of the invention comprise compounds, compositions, methods useful for the treatment of drug addiction, drug withdrawal, and diseases or conditions that involve dysregulation of glutamate homeostasis in it etiology.
Owner:TEMPLE UNIVERSITY
Pharmaceutical composition for preventing dysbiosis associated with enteral administration of antibiotics
InactiveUS20110200668A1Increase rangeEliminate side effectsAntibacterial agentsPowder deliveryIsomaltooligosaccharideAntibiotic Y
In the first variant, the pharmaceutical composition comprises antibiotic and prebiotic in the form of oligosaccharide of a group comprising fructooligosaccharides, galactooligosaccharides, maltooligosaccharides and isomaltooligosaccharides the polymerisation degree of which ranges from 2 to 10, the particle size is equal to less than 0.3 mm and the purity is of at least 95%, wherein the antibiotic particle size ranges from 20 to 200 mkm and the antibiotic and oligosaccharide are contained with a mass ratio ranging from 1:1 to 1:100 respectively. In the second variant, the pharmaceutical composition comprises antibiotic which is in the form of a powder with the particle size ranging from 20 t0 200 mkm and is selected form a group consisting of beta-lactams, including the combination thereof with bacterial betalactamase inhibitors, azalides, fluoroquinalones, amphenicols, glycopeptides, ansamycins, nitrofurans, phosphonic acid derivatives, cycloserine and trimetoprim. The prebiotic is in the form of a powder oligosaccharide, the polymerisation degree of which ranges from 2 to 10, the particle size is equal to less than 0.3 mm and the purity is of at least 95%, wherein the antibiotic and oligosaccharide are contained with a mass ratio ranging from 1:1 to 1:100 respectively. The above-mentioned compositions are used for preventing intestinal dysbiosis during antibiotic therapy and are perorally administered. The inventive composition produces a stimulating effect on intestinal lactobacilli and bifidobacteria simultaneously inhibiting the growth and proliferation of extraneous or pathogenic microflora.
Owner:DIKOVSKIY ALEKSANDER VLADIMIROVICH +2
Pharmaceutical composition for preventing dysbiosis associated with enteral administration of antibiotics
In the first variant, the pharmaceutical composition comprises antibiotic and prebiotic in the form of oligosaccharide of a group comprising fructooligosaccharides, galactooligosaccharides, maltooligosaccharides and isomaltooligosaccharides the polymerisation degree of which ranges from 2 to 10, the particle size is equal to less than 0.3 mm and the purity is of at least 95%, wherein the antibiotic particle size ranges from 20 to 200 mkm and the antibiotic and oligosaccharide are contained with a mass ratio ranging from 1:1 to 1:100 respectively. In the second variant, the pharmaceutical composition comprises antibiotic which is in the form of a powder with the particle size ranging from 20 t0 200 mkm and is selected form a group consisting of beta-lactams, including the combination thereof with bacterial betalactamase inhibitors, azalides, fluoroquinalones, amphenicols, glycopeptides, ansamycins, nitrofurans, phosphonic acid derivatives, cycloserine and trimetoprim. The prebiotic is in the form of a powder oligosaccharide, the polymerisation degree of which ranges from 2 to 10, the particle size is equal to less than 0.3 mm and the purity is of at least 95%, wherein the antibiotic and oligosaccharide are contained with a mass ratio ranging from 1:1 to 1:100 respectively. The above-mentioned compositions are used for preventing intestinal dysbiosis during antibiotic therapy and are perorally administered. The inventive composition produces a stimulating effect on intestinal lactobacilli and bifidobacteria simultaneously inhibiting the growth and proliferation of extraneous or pathogenic microflora.
Owner:亚历山大·弗拉基米罗维奇·季科夫斯基
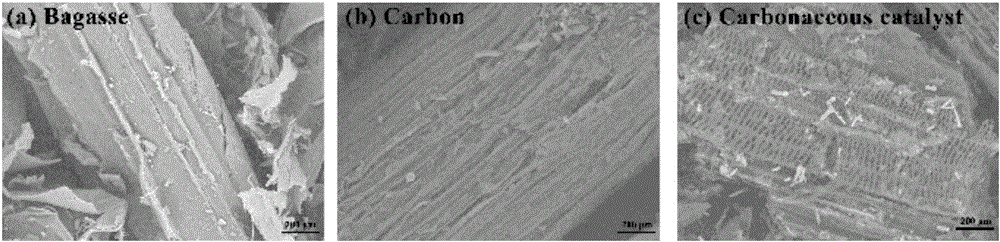
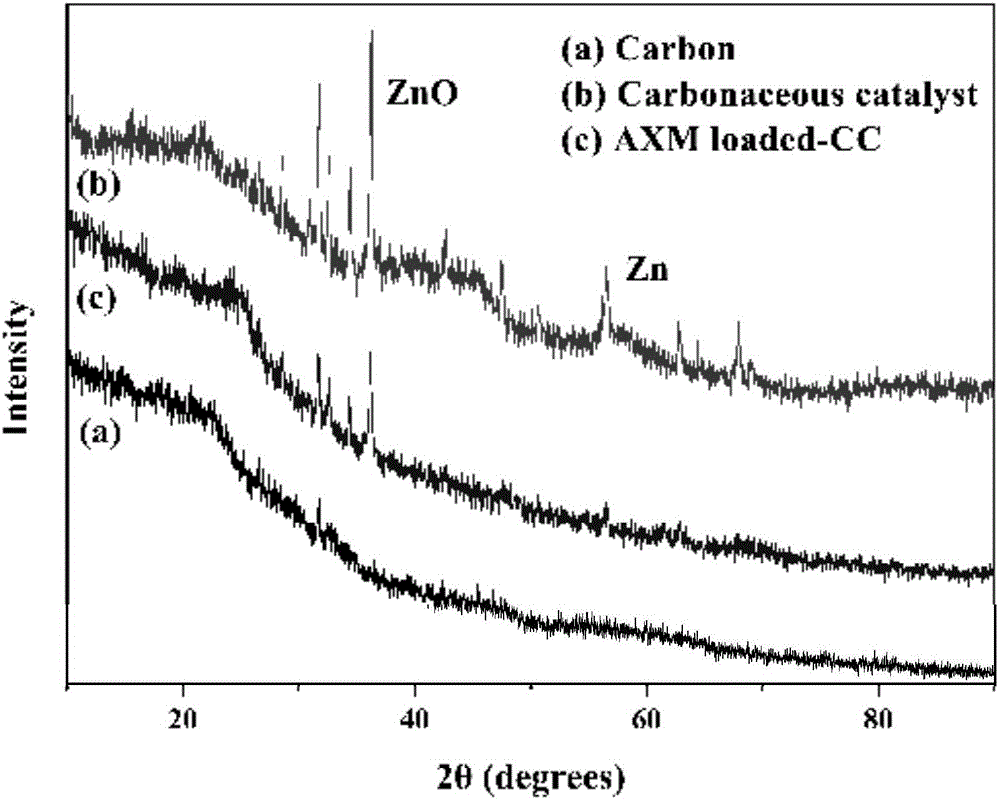
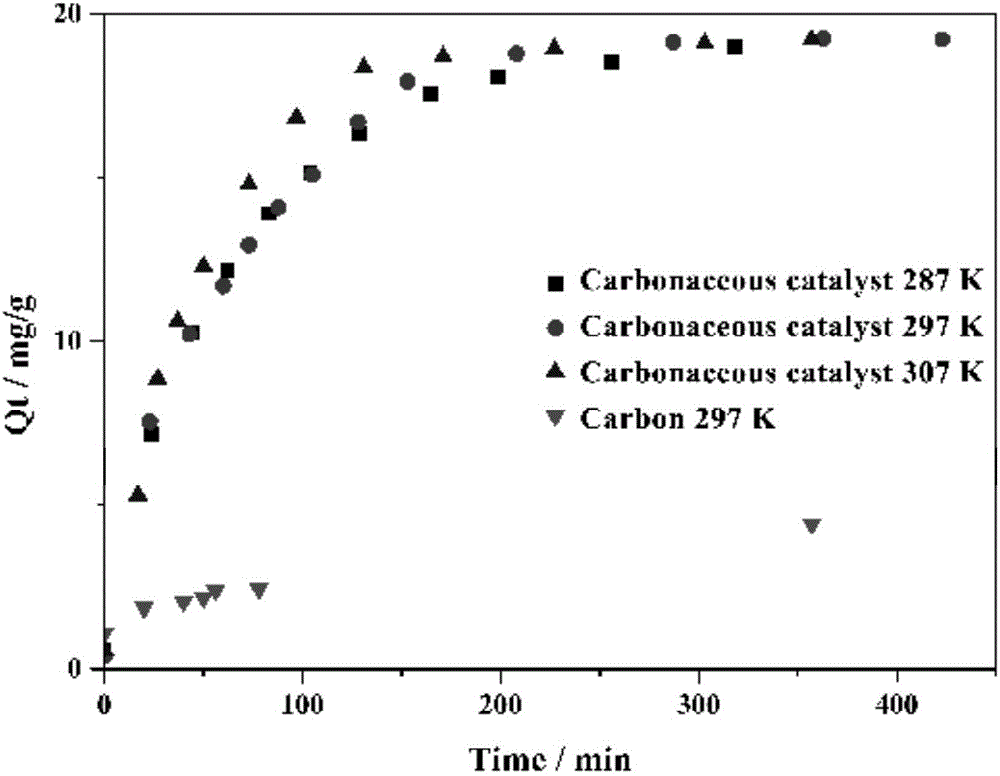
Bagasse-based carbon catalyst, as well as preparation method and application thereof
InactiveCN107519855APromote degradationLow equipment requirementsWater contaminantsCatalyst activation/preparationChlorideZinc
The invention provides a bagasse-based carbon catalyst, as well as a preparation method and application thereof. The catalyst is prepared by using bagasse as a raw material through a process of charring, activating and re-charring. The preparation method comprises the following steps: washing and drying bagasse, heating to a certain temperature in a charring environment, keeping the temperature for a period, naturally cooling, and keeping inert gas to flow in the whole process to obtain charred bagasse; mixing the charred bagasse with a zinc chloride solution, stirring and activating for a period of time at a certain temperature, and drying and storing the activated charred bagasse; heating the activated charred bagasse to a certain charring temperature in a charring environment at a certain heating speed, keeping the temperature for a period of time, stopping heating, naturally cooling, and keeping the inert gas to flow in the whole process to finally obtain a finished product bagasse-based carbon catalyst. The bagasse-based carbon catalyst has an extremely good effect for penicillin broad spectrum beta-lactams in a water body; and the preparation method is simple, raw materials are low in cost.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Antibiotic combinations for providing total solution to the treatment of infections
The invention relates to a new pharmaceutical composition, a method of treatment of infection and also a process to prepare the composition. The infectious complications are important causes of morbidity and mortality. Hospital acquired pneumonia (HAP) remains the most severe nosocomial infection in intensive care units. Beta-lactams alone are always considered inadequate when P. aeruginosa and / or methicillin-resistant S. aureus are implicated as pathogens or copathognes. The present invention provides the desired empirical therapy for control of all bacterial infections. The invention provides antibiotic combination products for delivering at least two different antibiotics, through parenteral dosage form comprising protein-synthesis-inhibiting antibiotic which is amikacin or its sulphate salt and non-protein-synthesis-inhibiting antibiotic which is cefepime or its hydrochloride salt. The invention provides a total solution, against multiresistant P. aeruginosa, or Acinetobacter spp. and / or methicillin-resistant S. aureus, and are useful for intramuscular or intravenous administration as antibiotics for hospitalized patients with acute or serious infections. The pharmaceutical compositions described here normally have the least nephrotoxicity and have better efficacy and safety of cefepime plus amikacin combination.
Owner:VENUS REMEDIES LTD
Beta lactams as modulators of glutamate uptake and methods for use thereof
Pharmaceutical compositions of the invention comprise compounds, compositions, methods useful for the treatment of drug addiction, drug withdrawal, and diseases or conditions that involve dysregulation of glutamate homeostasis in its etiology.
Owner:TEMPLE UNIVERSITY
Methods for improved in vitro - in vivo efficacy determination
The invention provides methods for determining and evaluating the in vitro-in vivo activity relationship of the efficacy of families of compounds for infectious diseases such as tuberculosis. The validity of the methods can be confirmed by evaluation of the compounds in animal models, for example, in murine models of tuberculosis. Examples of families of antibacterial compounds that can be evaluated for in vivo efficacy using the in vitro methods described herein include benzimidazoles, pyridopyrazines, pteridines, diphenyl ethers, beta-lactams, PBP inhibitors, and compounds that are non-ribonucleic acid and protein synthesis inhibitors. The methods can be used to evaluate classes of small molecule compounds and inhibitors that may be effective against any bacterial pathogen. The methods aid the identification of compounds, such as various benzimidazoles, with modes of action having activity against clinical isolates, as well as non-replicating persistent bacilli, which can therefore enhance current clinical therapeutic regimens.
Owner:COLORADO STATE UNIVERSITY
Therapeutic beta-lactams
Compounds comprisingor a pharmaceutically acceptable salt or a prodrug thereof, are disclosed, wherein Y isA is —(CH2)6—, cis —CH2CH═CH—(CH2)3—, or —CH2C≡C—(CH2)3—, wherein 1 or 2 carbon atoms may be substituted with S or O; or A is —(CH2)m—Ar—(CH2)o— wherein Ar is substituted or unsubstituted phenyl or monocyclic heteroaryl, the sum of m and o is from 1 to 4, and wherein one CH2 may be replaced with S or O; X is S or O; R is a hydrocarbyl or a hydroxyhydrocarbyl moiety having from 1 to 12 carbon atoms; D is independently a moiety comprising from 1 to 6 non-hydrogen atoms; and n is an integer from 0 to 4. Methods, compositions, and medicaments related thereto are also disclosed.
Owner:ALLERGAN INC
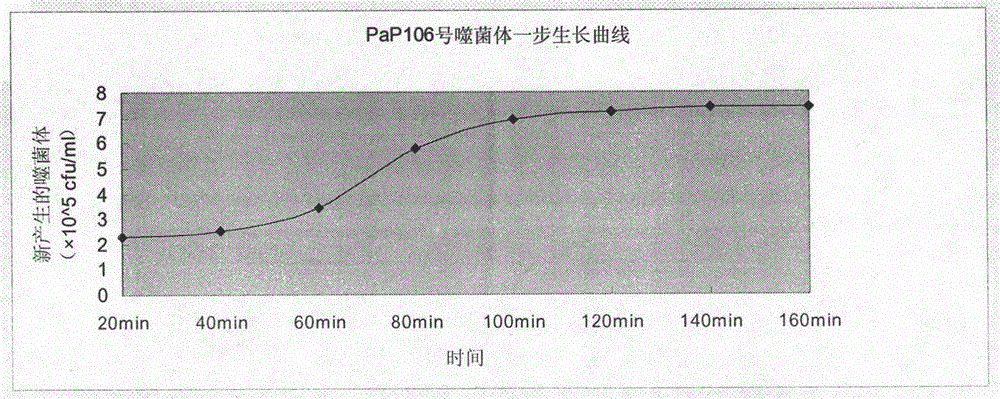
A phage capable of lysing multidrug-resistant Pseudomonas aeruginosa and its application in treating infection
InactiveCN104140957BStrong cracking abilityImprove the bactericidal effectAntibacterial agentsViral/bacteriophage medical ingredientsAntibiotic YAnti-infective therapy
The invention discloses cleavable multiple-drug resistant pseudomonas aeruginosa bacteriophage and application thereof in infection treatment. The invention discloses cleavable pseudomonas aeruginosa bacteriophage multiple-resistant to beta-lactams, aminoglycosides and cephalosporin antibiotics, which is named as PaP No.106, preserved in China Center for Type CultureCollection(CCTCC) (Budapest treaty preservation unit, Wuhan University, Wuhan City, China) in June 1, 2012 and has the preservation number of CCTCC NO:M2012200. On the basis of the purpose of novel anti infective therapy, the separated and purified cleavable multiple-drug resistant pseudomonas aeruginosa bacteriophage is proved to be capable of cleaving into multi strains of pseudomonas aeruginosa multiple-resistant to beta-lactams (including carbapenems), aminoglycosides and cephalosporin antibiotics in vitro and vivo, so that the cleavable multiple-drug resistant pseudomonas aeruginosa bacteriophage is proved to be feasible in treatment of pseudomonas aeruginosa infection, and a new therapeutic approach for clinical drug resistant bacteria infection is provided.
Owner:LANZHOU UNIVERSITY
Liposomal formulations of amidine substituted beta-lactam compounds for use in the treatment of bacterial infections
InactiveCN109982696AAvoid damageLow toxicityAntibacterial agentsOrganic active ingredientsMedicineCompound (substance)
Owner:AICURIS ANTI INFECTIVE CURES
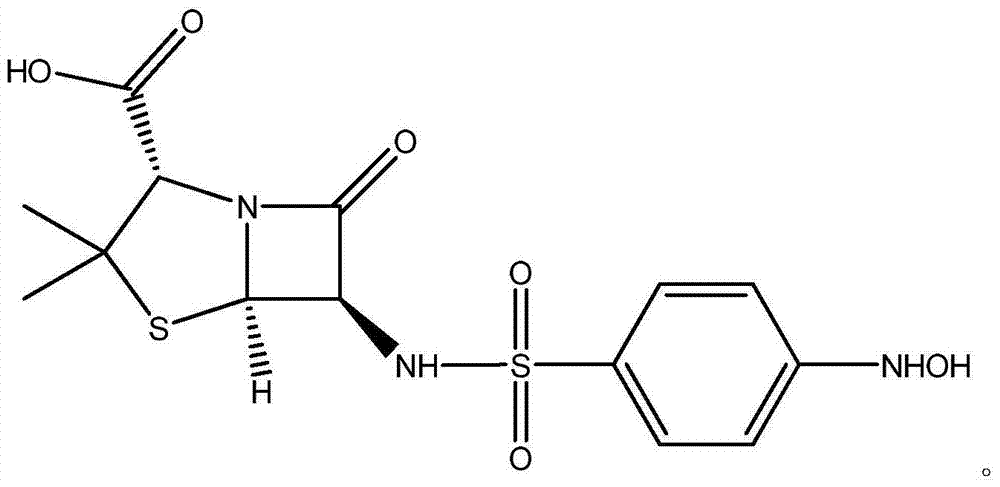
Beta-lactam compound as well as preparation method and application of beta-lactam compound
InactiveCN103965214AMedicinalHigh antibacterial activityAntibacterial agentsOrganic chemistrySulfur drugSide effect
The invention discloses a beta-lactam compound, and the chemical structural formula of the beta-lactam compound is shown in the specification. The invention also discloses a preparation method and an application of the beta-lactam compound. The beta-lactam compound disclosed by the invention, which has a feature pharmacophores (p-aminobenzenesulfonamide) of sulfonamides drugs and a feature pharmacophore (6-APA or 7-ADCA) of beta-lactams drugs, is obtained by synthesizing on the basis of combination principles. Since the pharmacophores of both drugs are compatible in a molecule, the compound disclosed by the invention has not only the drug properties of sulfonamides drugs and beta-lactams drugs, but also a synergistic effect between two pharmacophores, and thus the antibacterial activity of the compound disclosed by the invention is effectively enhanced and the toxic and side effect of drugs on the human body is reduced. The preliminary studies show that the compound disclosed by the invention exhibits good antibacterial activity against Pseudomonas aeruginosa and is comparable to carbenicillin.
Owner:SOUTHEAST UNIV
Novel beta lactams as modulators of glutamate uptake and methods for use thereof
Pharmaceutical compositions of the invention comprise compounds, compositions, methods useful for the treatment of drug addiction, drug withdrawal, and diseases or conditions that involve dysregulation of glutamate homeostasis in it etiology.
Owner:TEMPLE UNIVERSITY
Pharmaceutical composition and application thereof
InactiveCN108392479AAvoid infectionAddressing drug resistanceAntibacterial agentsTetracycline active ingredientsDiseaseSide effect
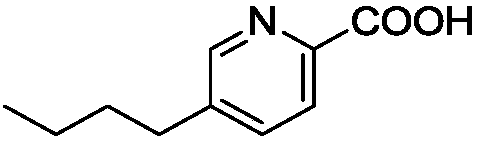
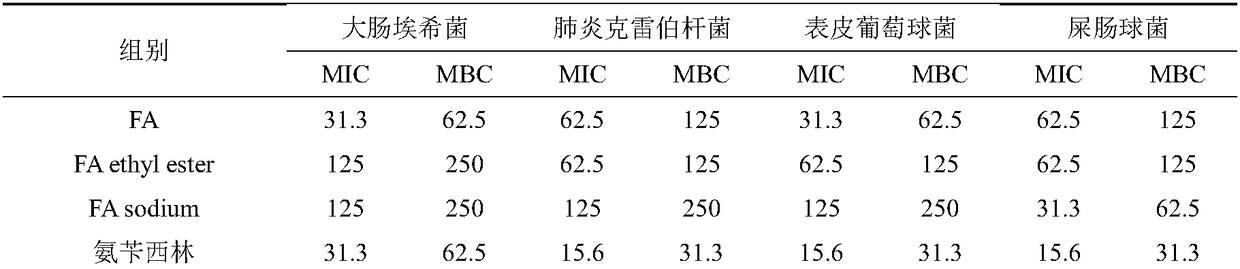
The invention discloses a pharmaceutical composition and an application thereof, which belongs to the technical field of a medicine. The pharmaceutical composition comprises fusaric acid or a fusaricacid compound and one of quinolone, beta-lactams and / or tetracycline antibiotic or a plurality of antibiotics with a mass ratio of 1: 500-128:1, and is used for preparing a medicine for treating and preventing diseases due to drug-resistant bacteria. The composition can effectively solve the drug resistance problem of bacteria on a plurality of antibiotics, the components have synergistic interaction effect, the usage of antibiosis drugs in clinical application of the antibiosis drugs is reduced, side effect and the treatment cost generated due to antibiotic can be correspondingly reduced, andthe sensitivity of the antibiosis drugs can be reduced.
Owner:GUIZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com