Beta-lactam compound as well as preparation method and application of beta-lactam compound
A technology for lactams and compounds, which is applied in the field of preparation of the above-mentioned β-lactam compounds, can solve the problems of antibacterial reduction and other problems, and achieve the effect of enhancing antibacterial activity and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
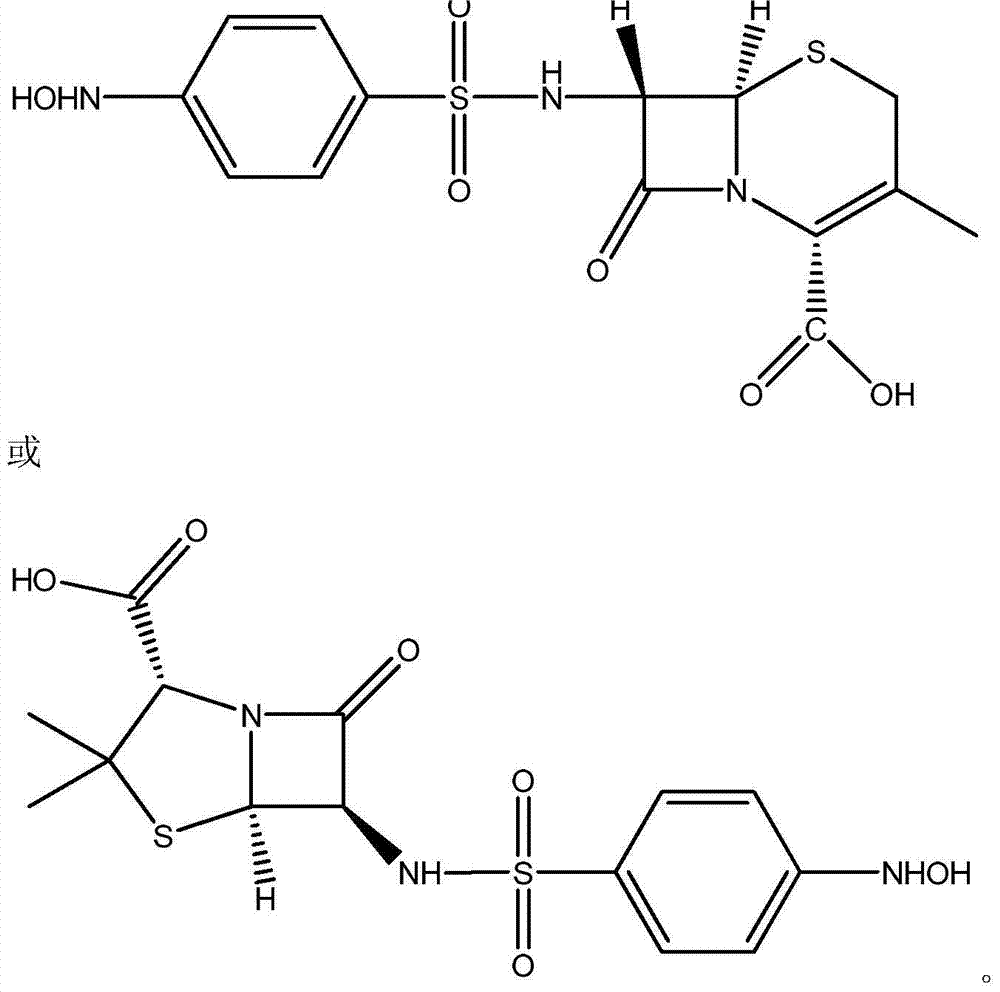
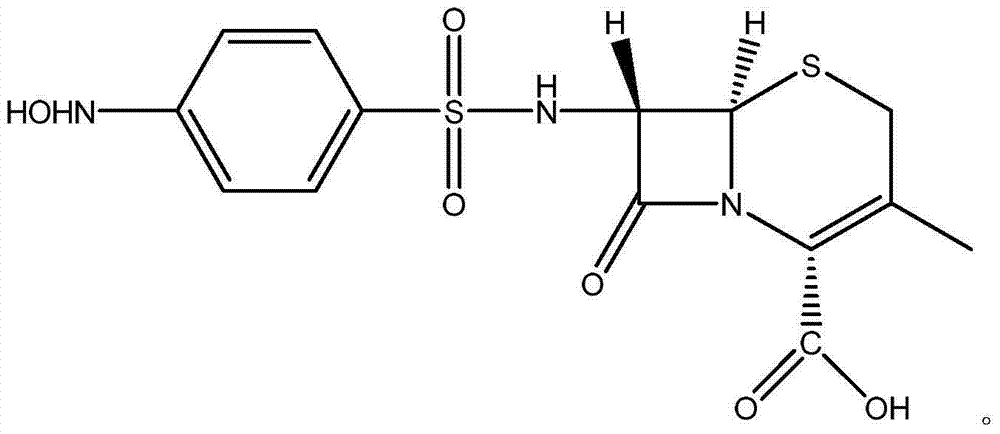
[0021] A kind of preparation method of 7-(4-parahydroxylaminobenzenesulfonyl)-3-desacetoxy cephalosporanic acid, adopts following steps to obtain:
[0022] Add 10ml of water, 10ml of acetone and 30ml of 0.76mol / L NaHCO to the four-necked flask in sequence 3 Solution, after being cooled to-5 ℃, add 2.14g7-ADCA (10mmol) again, carry out reaction; After reacting for half an hour until the solution is clear, keep the temperature constant, slowly drop 4.42g p-nitrobenzenesulfonyl chloride ( 0.02mol), reacted for 3h and filtered; at room temperature, distilled off the acetone in the filtrate, washed the solution with 30ml ethyl acetate for 3 times; then transferred the solution to a 200ml beaker, and added 50ml Ethyl acetate, and then drop an appropriate amount of saturated oxalic acid solution to adjust the pH value of the solution. When the pH of the solution is 2-3, separate the organic phase; wash the organic phase with 10ml saturated saline for 3 times, and then wash the organi...
Embodiment 2
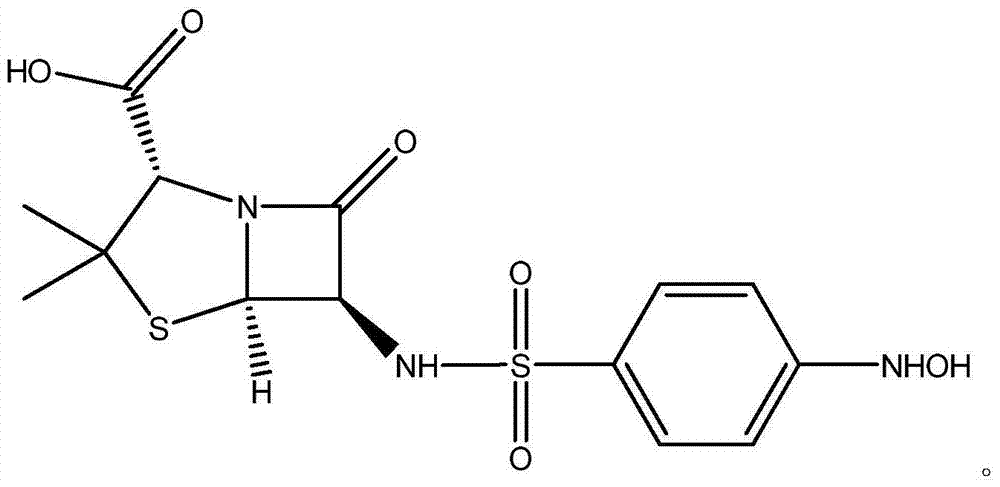
[0026] A kind of preparation method of 6-(4-parahydroxylaminobenzenesulfonyl) penicillanic acid, adopts following steps to obtain:
[0027] Add 10ml of water, 10ml of acetone and 30ml of 0.76mol / L KHCO to the four-necked flask in sequence 3 Solution, after being cooled to-5 ℃, add 2.14g6-APA (10mmol) again, carry out reaction; After reacting half an hour to solution clarification, keep temperature constant, in solution, slowly drop into 4.42g p-nitrobenzenesulfonyl chloride ( 0.02mol), reacted for 3h and filtered; at room temperature, distilled off the acetone in the filtrate, washed the solution with 30ml ethyl acetate for 3 times; then transferred the solution to a 200ml beaker, and added 50ml Ethyl acetate, then add dropwise an appropriate amount of saturated oxalic acid solution to adjust the pH value of the solution, when the pH of the solution is 2-3, carry out liquid separation operation; the organic phase after liquid separation is washed 3 times with 10ml saturated sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com