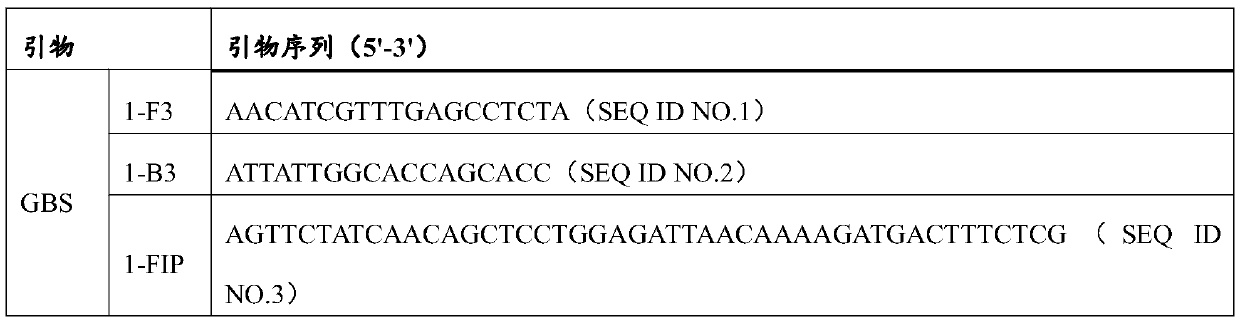
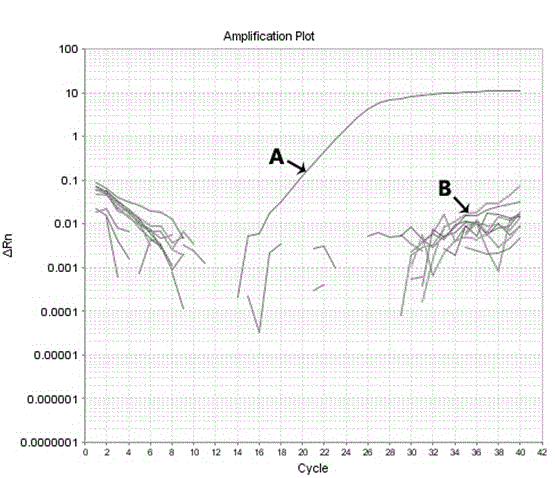
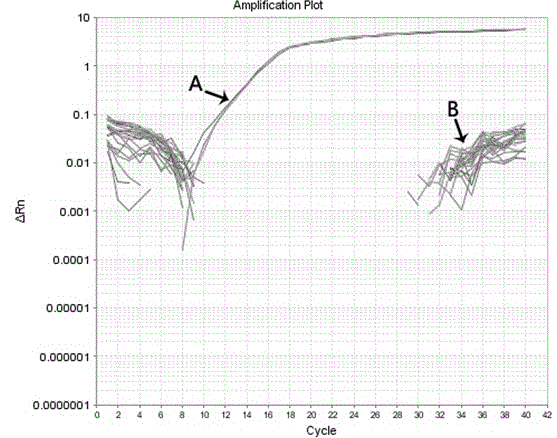
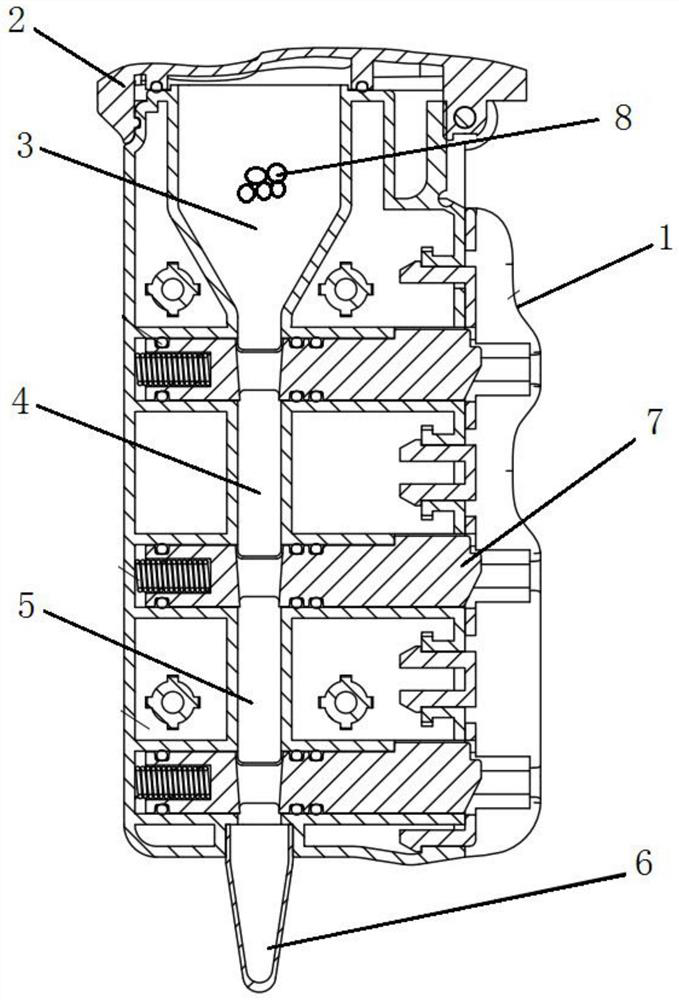
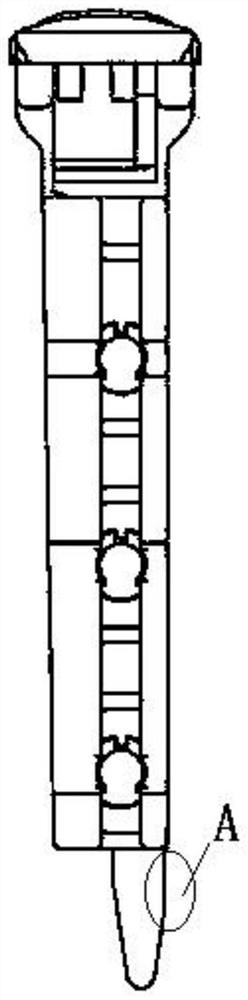
Patents
Literature
40 results about "Chlamydiaceae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
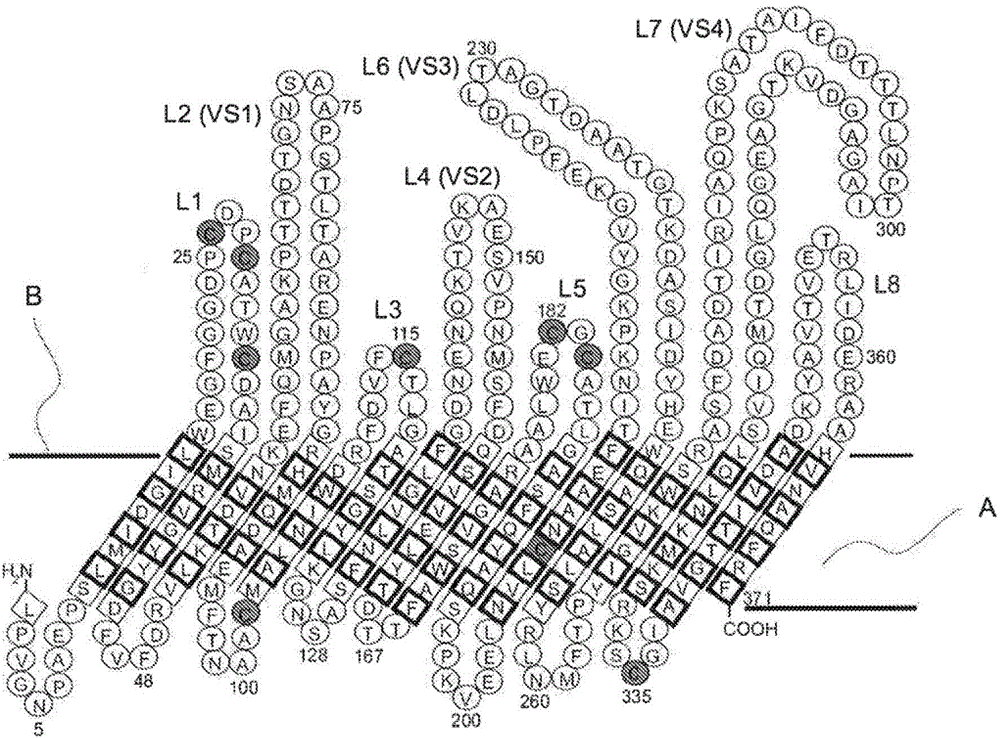
The Chlamydiaceae are a family of gram-negative bacteria that belongs to the phylum Chlamydiae, order Chlamydiales. Chlamydiaceae species express the family-specific lipopolysaccharide epitope αKdo-(2→8)-αKdo-(2→4)-αKdo (previously called the genus-specific epitope). Chlamydiaceae ribosomal RNA genes all have at least 90% DNA sequence identity. Chlamydiaceae species have varying inclusion morphology, varying extrachromosomal plasmid content, and varying sulfadiazine resistance.
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Chlamydia trachomatis specific peptides and their use in diagnostic assays
InactiveUS6699678B1High sensitivityStrong specificityPeptide/protein ingredientsMicroorganismsAntiendomysial antibodiesDrug biological activity
Disclosed are peptides or a mixture of peptides, or analogs thereof, derived from the variable domains of the Chlamydia trachomatis (C. trachomatis) immunodominant major outer membrane protein (MOMP). The peptides or mixtures of peptides are characterized by having specificity only to C. trachomatis anti-MOMP antibodies and being non-cross reactive with anti-MOMP antibodies of other Chlamydia species Specific peptides are described (SEQ ID Nos. 1 to 8) as well as their analogs, which have essentially the same biological activity.
Owner:SAVYON DIAGNOSTICS
Methods and Compositions for Chlamydial Antigens for Diagnosis and Treatment of Chlamydial Infection and Disease
ActiveUS20110256094A1Reduce the possibilityReduce morbidityAntibacterial agentsBacterial antigen ingredientsAdjuvantTreatment chlamydia
Disclosed are isolated Chlamydia trachomatis proteins, methods of fusion protein and associated antibody production, and methods of using isolated proteins and antibodies in diagnosis and detection. Also disclosed are compositions comprising isolated proteins, wherein the compositions can further comprising pharmaceutically acceptable carriers, an adjuvant and / or an immunostimulant, and methods using the pharmaceutical compositions for treating or preventing an infection by Chlamydia in a subject. The compositions may also comprise a protein or immunogenic fragment of a pathogenic organism other than Chlamydia trachomatis.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Rapid-recognition gene chip for pathogenic bacteria of pneumonia
The invention discloses a rapid-recognition gene chip for pathogenic bacteria of pneumonia. The gene chip can detect 15 pathogenic bacteria including streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae, and clinically common and difficult-to-culture pathogenic bacteria are contained. In a preparation process of the gene chip, design, screening and verification of probes are performed by adopting 16S rDNA and a specific gene sequence corresponding to each of the pathogenic bacteria, and types of the bacteria in a to-be-detected sample are identified from levels of genus and species simultaneously and respectively. The gene chip has the advantages that the defect that clinical detection of the pathogenic bacteria of pneumonia is not timely and comprehensive at present is overcome, and one novel detection way is provided for early diagnosis and early treatment of patients with pneumonia.
Owner:GENERAL HOSPITAL OF PLA +1
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS20130023443A1Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
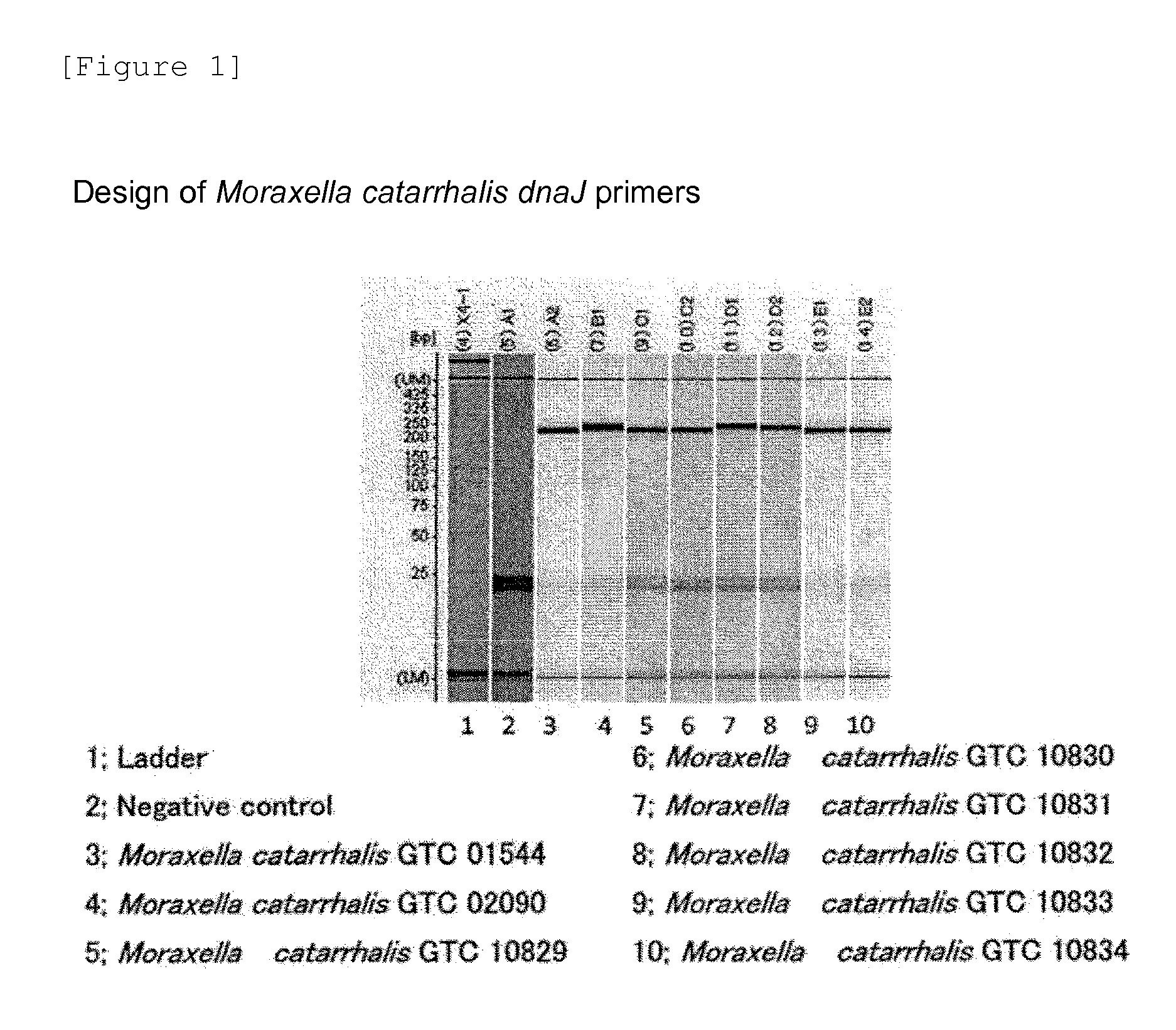
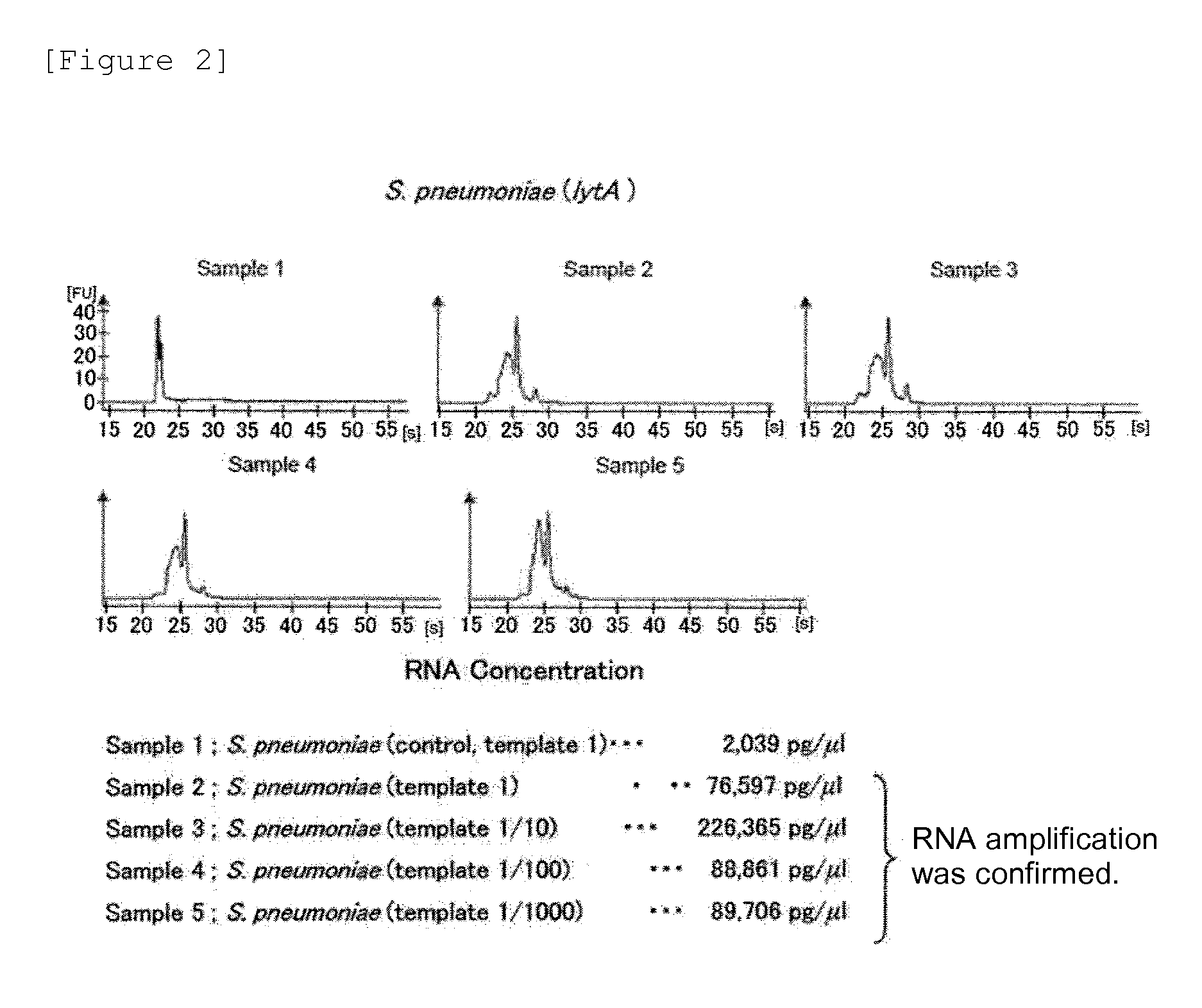
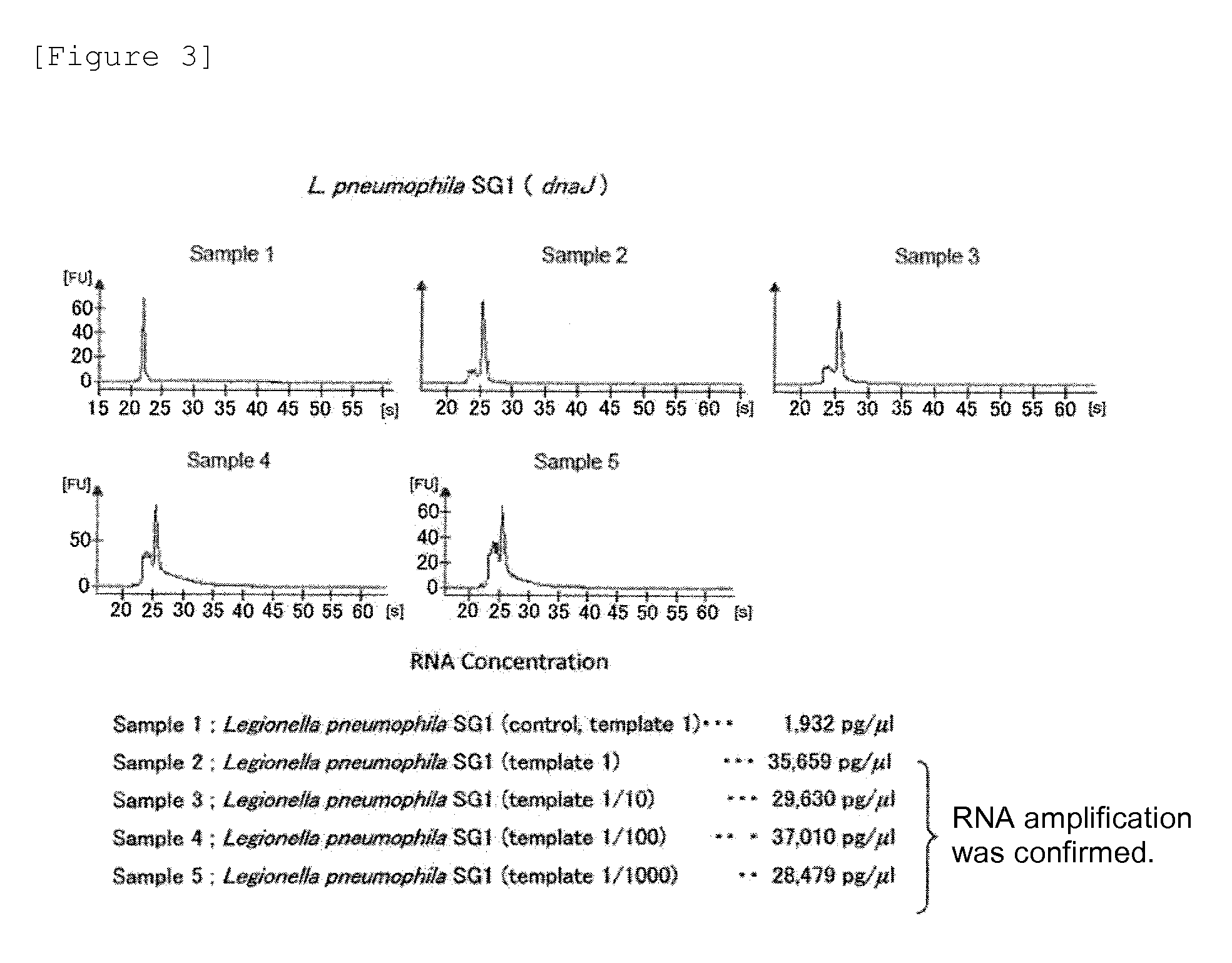
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
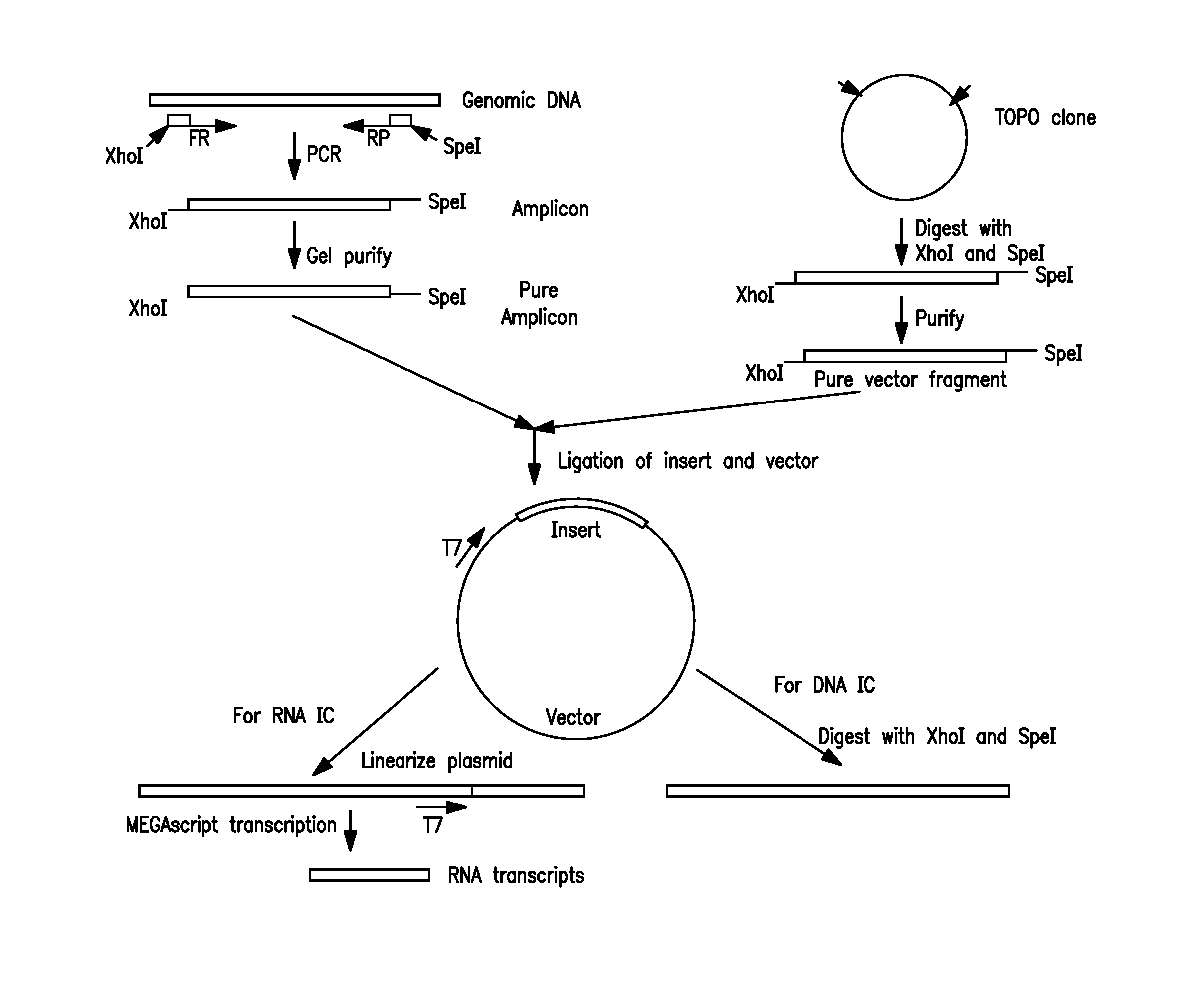
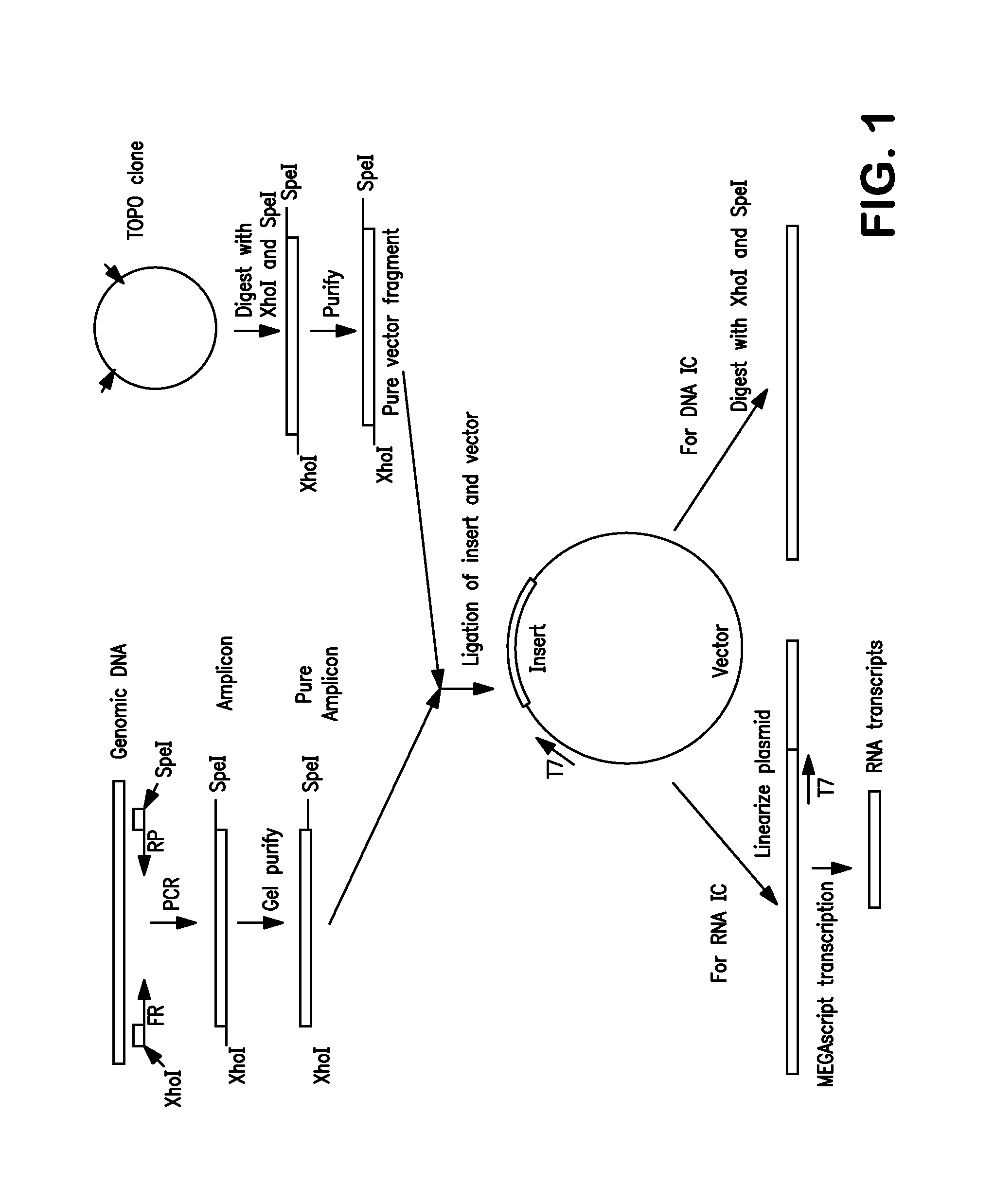
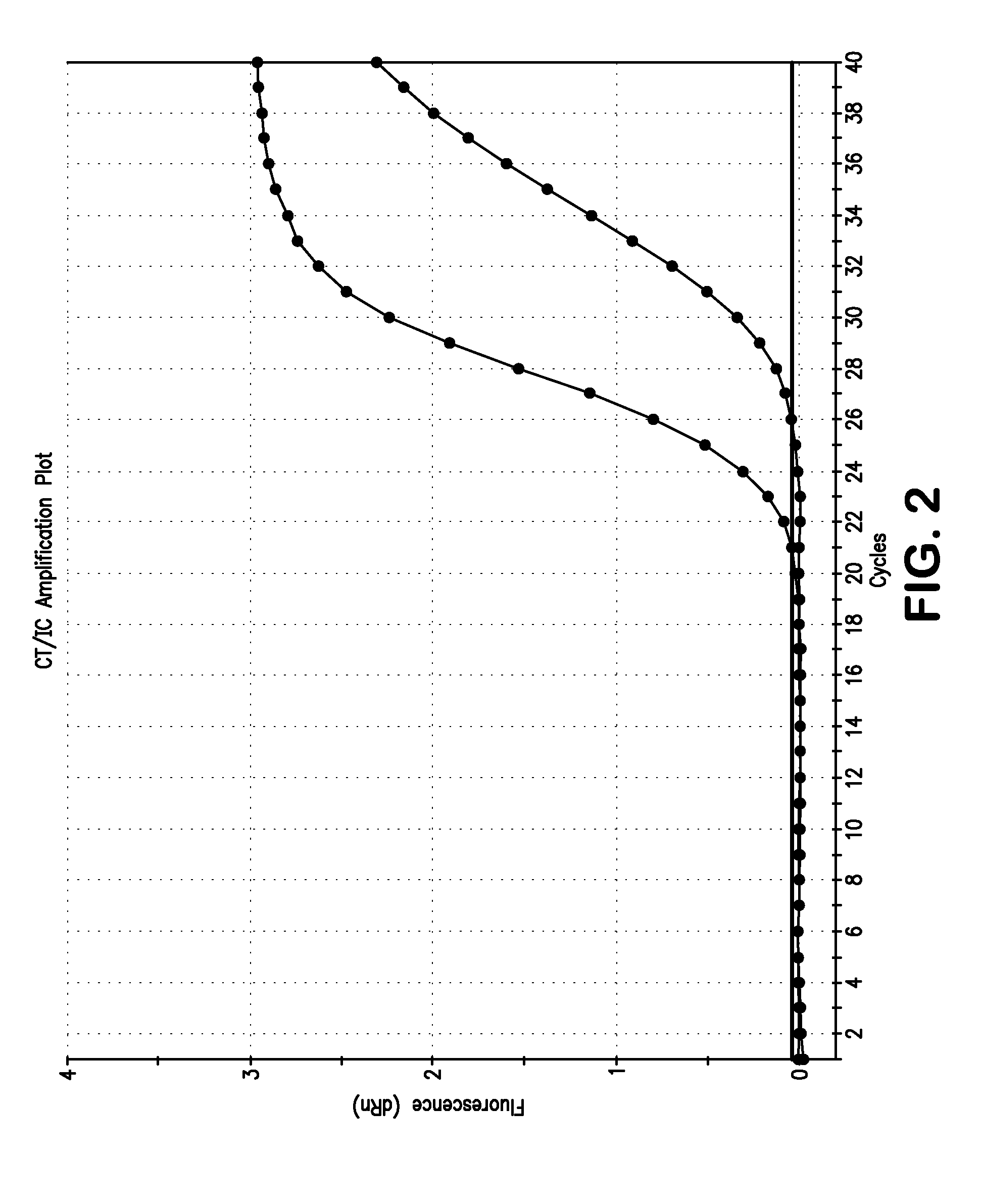
Non-Competitive Internal Controls for Use in Nucleic Acid Tests
InactiveUS20110003309A1Sugar derivativesMicrobiological testing/measurementNucleic acid testHuman immunodeficiency
Provided are non-competitive internal controls for use in nucleic acid tests (NATs), which are obtained from the organisms Methanobacterium thermoautrophicum (MET) and Zea mays (Corn). The non-competitive internal controls have utility in DNA and RNA NATs selected from Influenza A, Influenza B, parainfluenza viruses 1 to 4 (PIV-1 to PIV-4), respiratory syncytial virus type A (RSV A), RSV B, human metapneumovirus (hMPV), Chlamydia trachomatis (CT), and Neisseria gonorrhea (GC), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Human Immunodeficiency Virus I (HIV-1), and Severe Acute Respiratory Syndrome (SARS).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
LAMP (Loop-Mediated Isothermal Amplification) primer group, micro-fluidic chip and kit for detecting reproductive tract pathogenic microorganisms
ActiveCN111455075AAccurate detectionHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesStreptococcus mastitidisReproductive tract
The invention provides a LAMP (Loop-Mediated Isothermal Amplification) primer group, a micro-fluidic chip and a kit for detecting reproductive tract pathogenic microorganisms, and belongs to the technical field of reproductive tract infection detection. The LAMP primer group comprises a streptococcus agalactiae detection primer group, an enterococcus faecalis primer group, a gardnerella vaginosisprimer group, a Candida albicans primer group and a Chlamydia trachomatis primer group. The above LAMP primer group is fixed on the micro-fluidic chip. The LAMP primer group, the micro-fluidic chip and the kit can quickly, sensitively and accurately detect streptococcus agalactiae, enterococcus faecalis, gardnerella vaginosis, Candida albicans and Chlamydia trachomatis by high repeatability.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection kit and application thereof
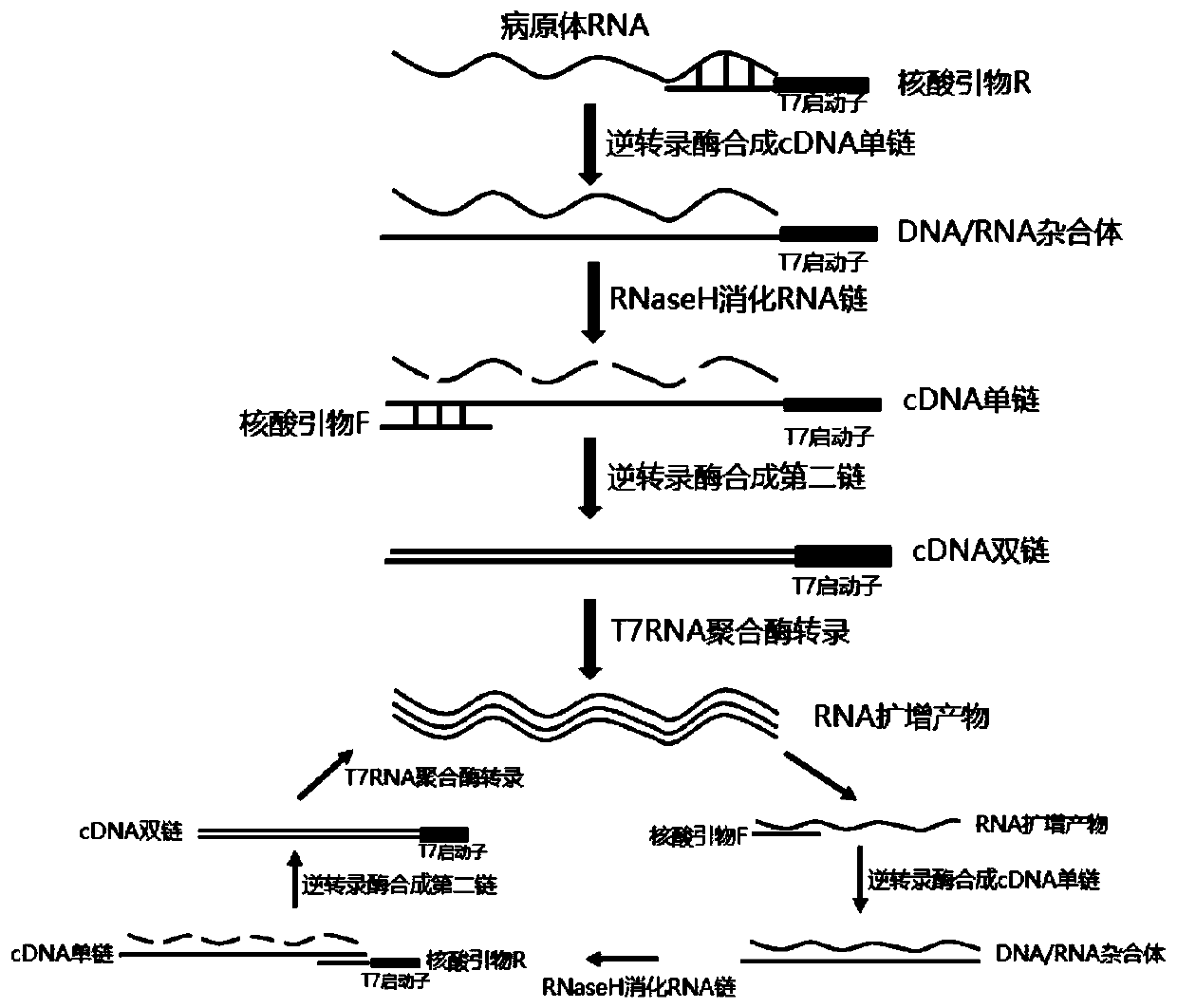
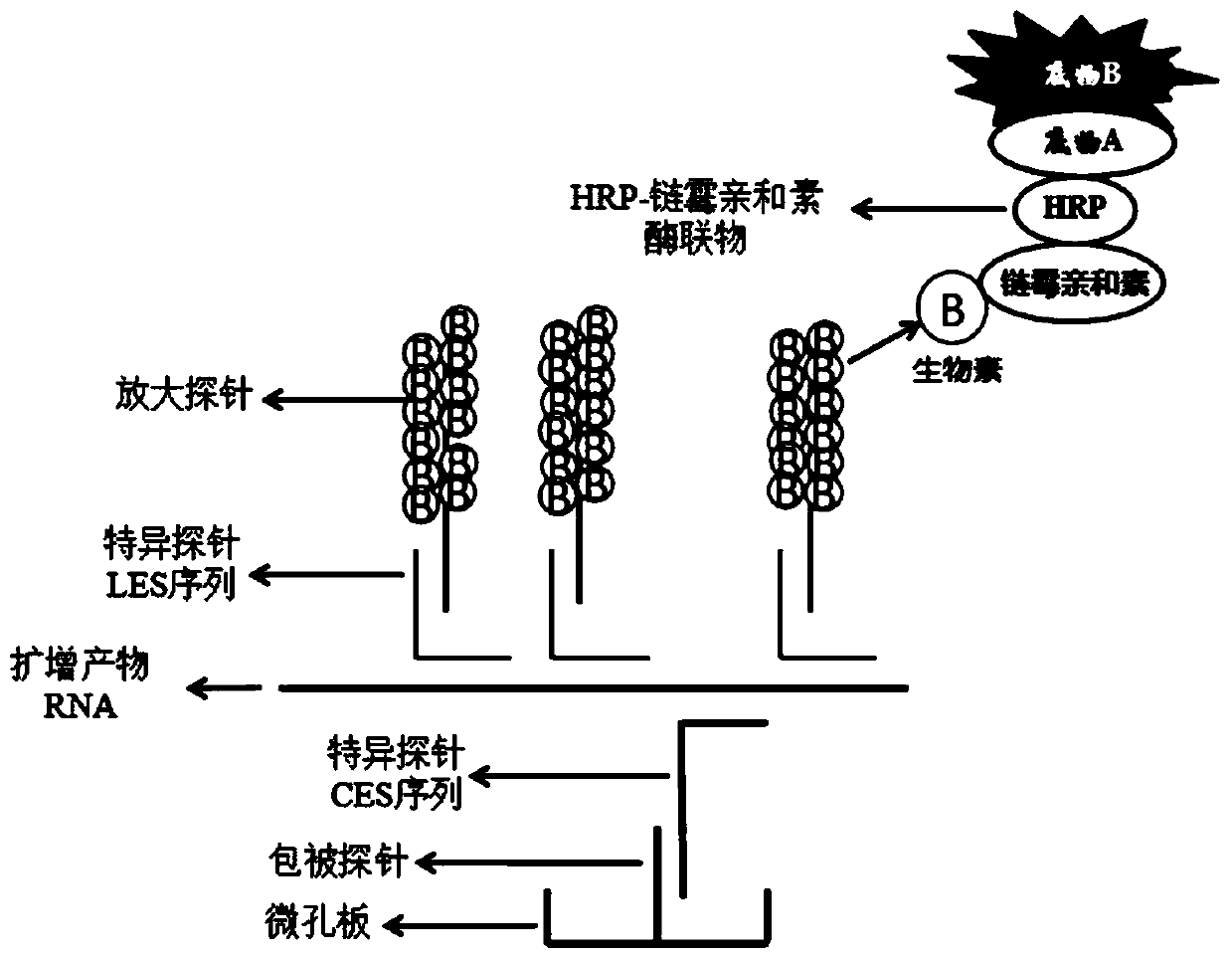
ActiveCN110904194AEasy to degradeLower requirementMicrobiological testing/measurementAgainst vector-borne diseasesRNA extractionNucleic acid detection
The invention discloses a mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection kit and an application thereof. A collected sample is split by a cell lysis solution to releasepathogen nucleic acid, and amplification of pathogen nucleic acid fragments is realized through reverse transcription and transcription processes. The amplified RNA product is added into a microporecoated with a coating probe, and a specific probe and an amplification probe are added at the same time, wherein the coating probe can be combined with one end of the specific probe CES to fix the amplified product RNA. One end of the specific probe LES is combined with the RNA product, and the other end of the specific probe LES is combined with the amplification probe to realize signal amplification. The amplification probe marked with multiple biotins is then combined with a streptavidin-HRP enzyme conjugate. Finally, an HRP enzyme chemiluminiscence substrate is added, and detection is carried out on a chemiluminiscence instrument. According to the invention, RNA extraction is not needed, and pollution is not likely to happen in the detection process; and the kit has the advantages of high sensitivity and high specificity, and can be widely applied to mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection.
Owner:武汉中帜生物科技股份有限公司
Chlamydia trachomatis recombinant protein and preparation method thereof
InactiveCN102925462AStrong specificityHigh sensitivityBacteriaMicroorganism based processesNucleotideImmunogenicity
The invention provides a chlamydia trachomatis recombinant protein and a preparation method thereof, relating to the field of gene engineering technology and diagnostic reagent and vaccine development. The invention relates to a chlamydia trachomatis fusion protein which can be applied to the clinical diagnosis of chlamydia trachomatis infection; and a nucleotide sequence coding the protein comprises plasmids of the nucleotide sequence and pronucleus host cells. The invention also relates to a method for preparing the protein and application thereof. The recombinant protein has high specificity and strong immunogenicity, and can improve the sensitivity and specificity of a detection reagent; and compared with the same kind of kits on the market, the recombinant protein has the advantages of strong specificity, high sensitivity and the like, and perfectly meets the needs in the clinical diagnosis of human chlamydia trachomatis infection.
Owner:英诺特(唐山)生物技术有限公司
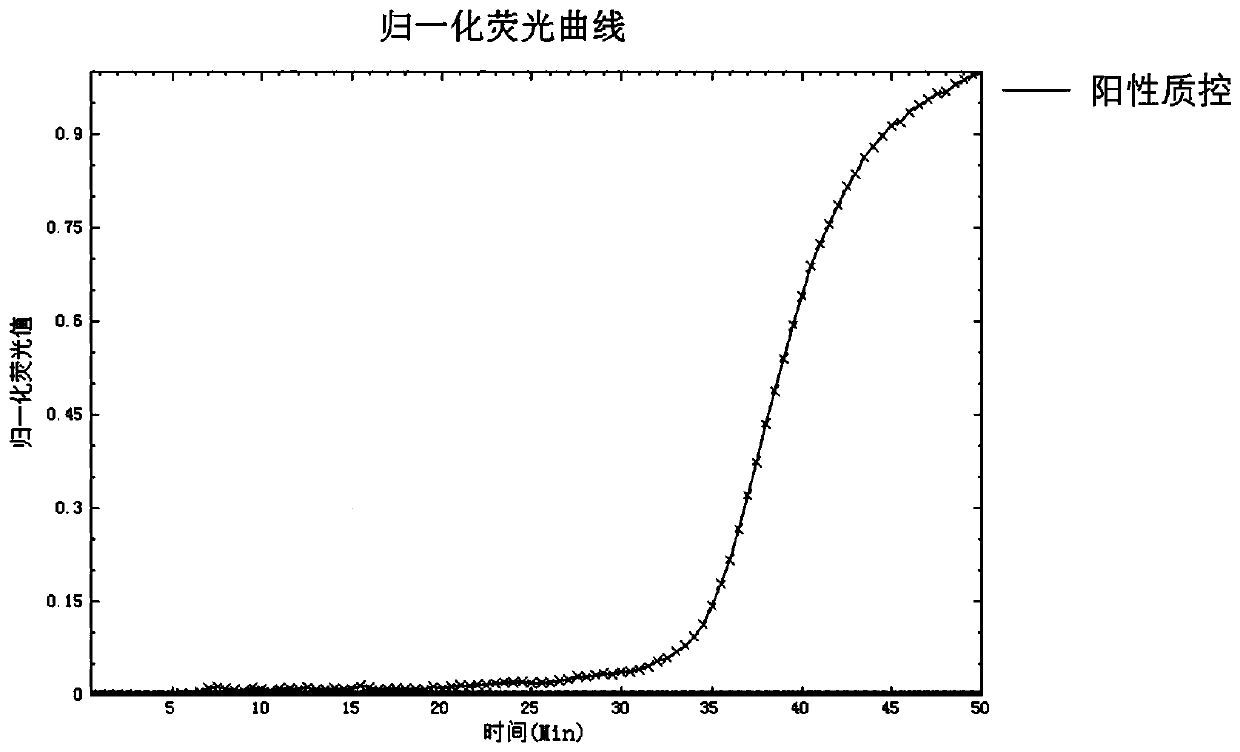
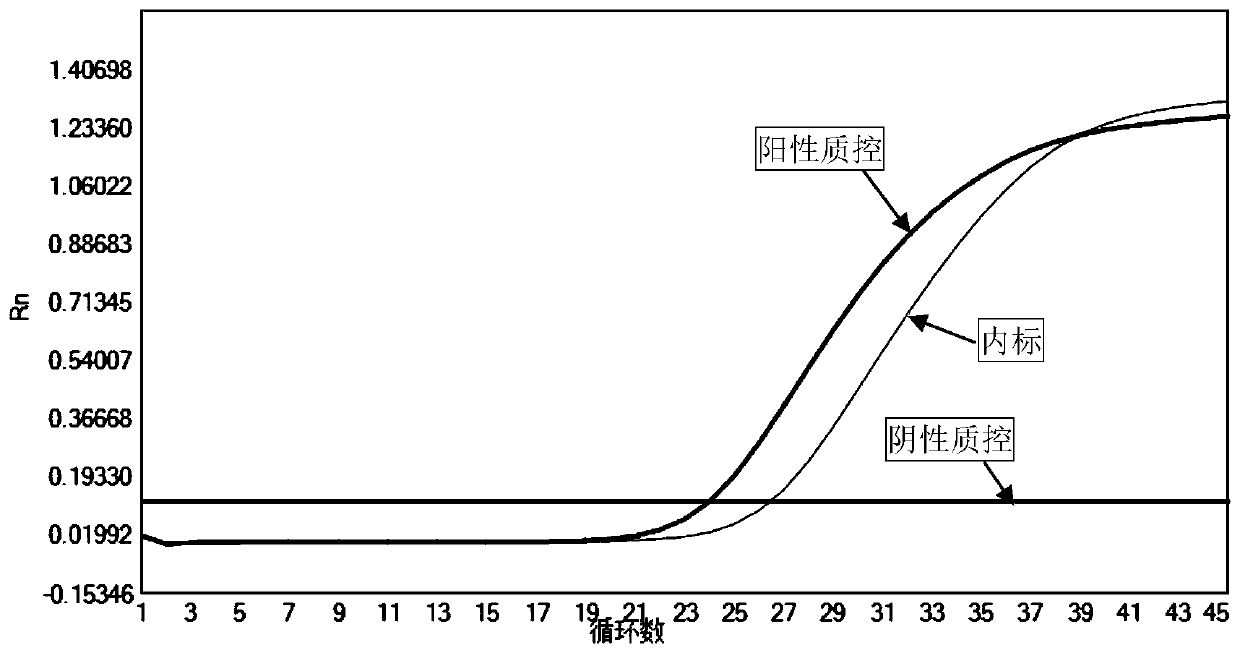
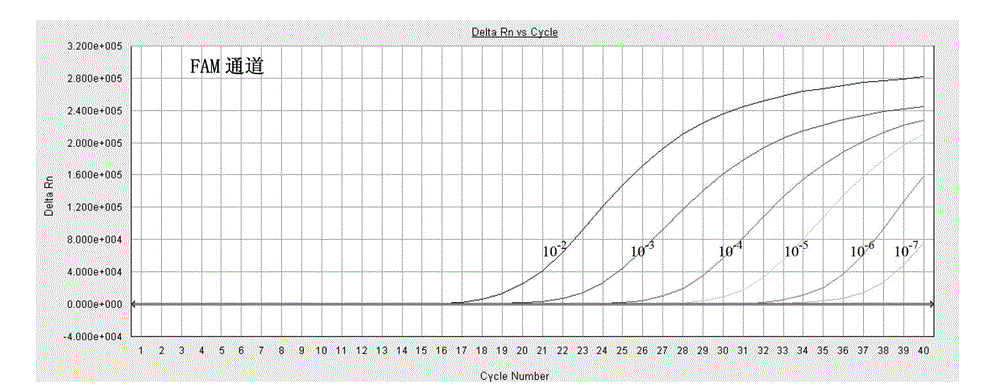
Fluorescent quantitative PCR detection kit for chlamydia trachomatis
InactiveCN105018573AStrong specificityShort detection timeMicrobiological testing/measurementFluoProbesA-DNA
The invention provides a fluorescent quantitative PCR detection kit for chlamydia trachomatis in clinic samples, used for assisted diagnosis of chlamydia trachomatis. The kit comprises a PCR solution, a DNA polymerase solution, positive quality control, weakly positive quality control, negative quality control, positive quantitative reference, lysate and protease K, wherein the PCR solution contains forward and reverse primers and the fluorescence probe are specific primers and probe designed for the specific sequence of chlamydia trachomatis and are capable of amplifying a target DNA sequence specifically so as to conveniently and quickly detect chlamydia trachomatis infection in clinic samples. The kit has the characteristics of high specificity and high sensitivity.
Owner:兰州安康伯乐生物技术有限公司
Chimeric momp antigen, method and use
ActiveCN103068837AEasy to produceFlexible route of administrationAntibacterial agentsAntibody mimetics/scaffoldsAntigenChlamydia trachomatis
The present invention regards polypeptides capable of eliciting an immunological response that is protective against Chlamydia trachomatis. The polypeptide comprises a first amino acid sequence which has at least 90% homology with the amino acid sequence according to SEQ ID NO: 1 and a second amino acid sequence which has at least 90% homology with the amino acid sequence according to SEQ ID NO: 2. Furthermore, production of these polypeptides and pharmaceutical compositions comprising them are also provided.
Owner:SPIXIA BIOTECH
Quintuple fluorescent PCR (polymerase chain reaction) quick and hypersensitive detection kit and application thereof
ActiveCN102888464BGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementFluorescence/phosphorescenceMycoplasma hominisFluorescent pcr
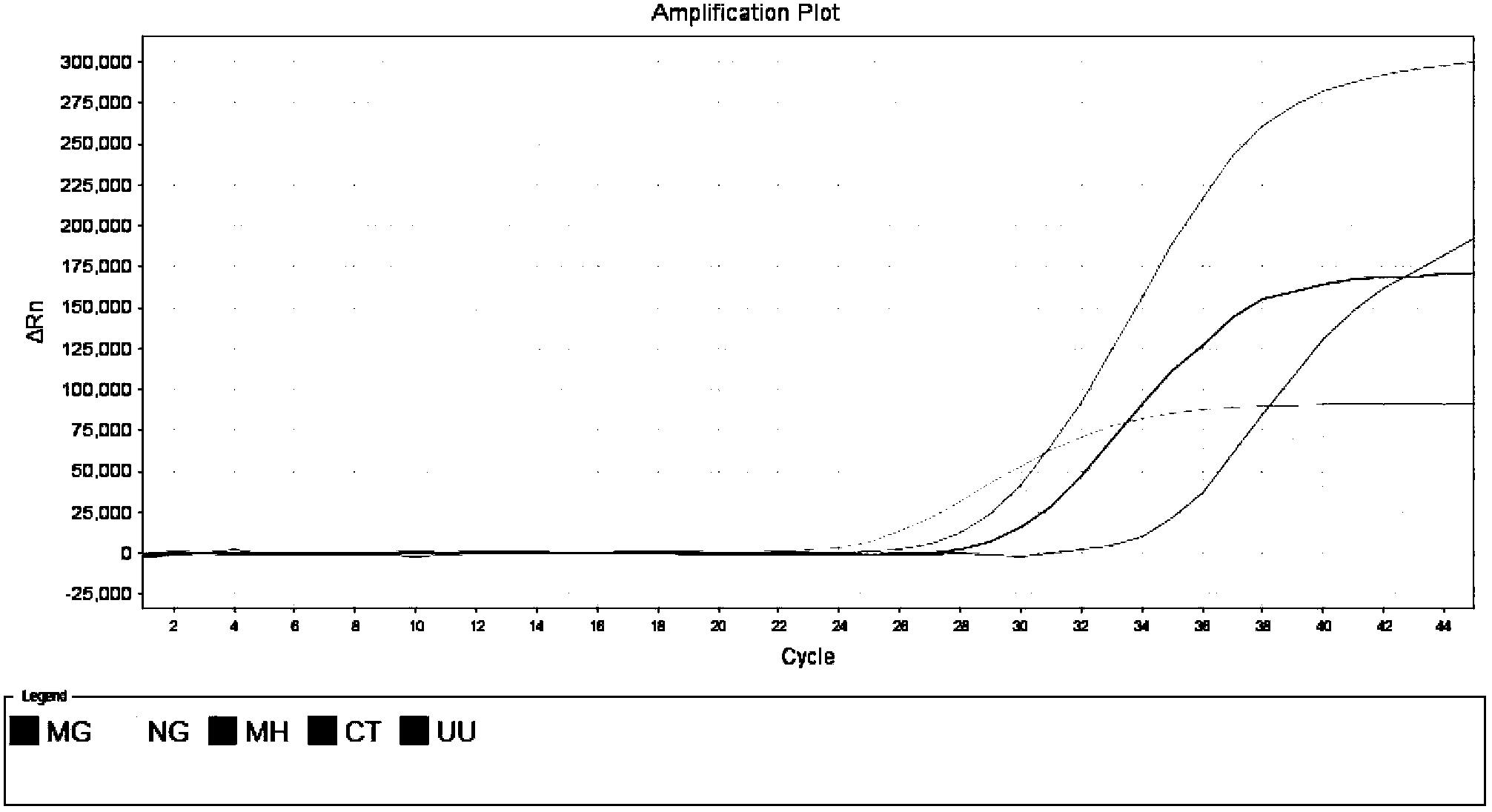
The invention relates to a real-time PCR (polymerase chain reaction) method for quintuply detecting target nucleic acid in a nucleic acid extracting solution in a single PCR reaction vessel, which is used for detecting Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in a sample.
Owner:苏州华益美生物科技有限公司
Hybridoma cell strain, and anti-chlamydia abortus monoclonal antibody secreted by hybridoma cell strain
ActiveCN108486066AHigh potencyStrong characteristicImmunoglobulins against bacteriaTissue cultureChlamydia abortusProkaryotic expression
The invention belongs to the field of immunology, and more specifically discloses a hybridoma cell strain, and an anti-chlamydia abortus monoclonal antibody secreted by the hybridoma cell strain. According to a preparation method, purified prokaryotically expressed chlamydia abortus major outer membrane protein (MOMP) is used for immunization of mouse as an antigen, indirect ELISA is adopted for screening of positive clones, and the hybridoma cell CA1-MM 01 capable of secreting the monoclonal antibodies aiming at chlamydia abortus major outer membrane protein is obtained. The identification ofthe monoclonal antibody in the aspects of the stability of the hybridoma cell after continuous continuous passage culture and cryopreservation, and the subtype and titer of the monoclonal antibody iscarried out, it is shown by results that the capacity of the hybridoma cell in secretion of antibodies is excellent and stable, the monoclonal antibody titer is high. It is shown that the obtained monoclonal antibody possesses extremely high specificity and combination capacity with chlamydia abortus.
Owner:CHINA AGRI UNIV
Method for detecting nucleic acids
InactiveUS20140017689A1Microbiological testing/measurementMaterial analysisChlamydia trachomatisNucleotide sequencing
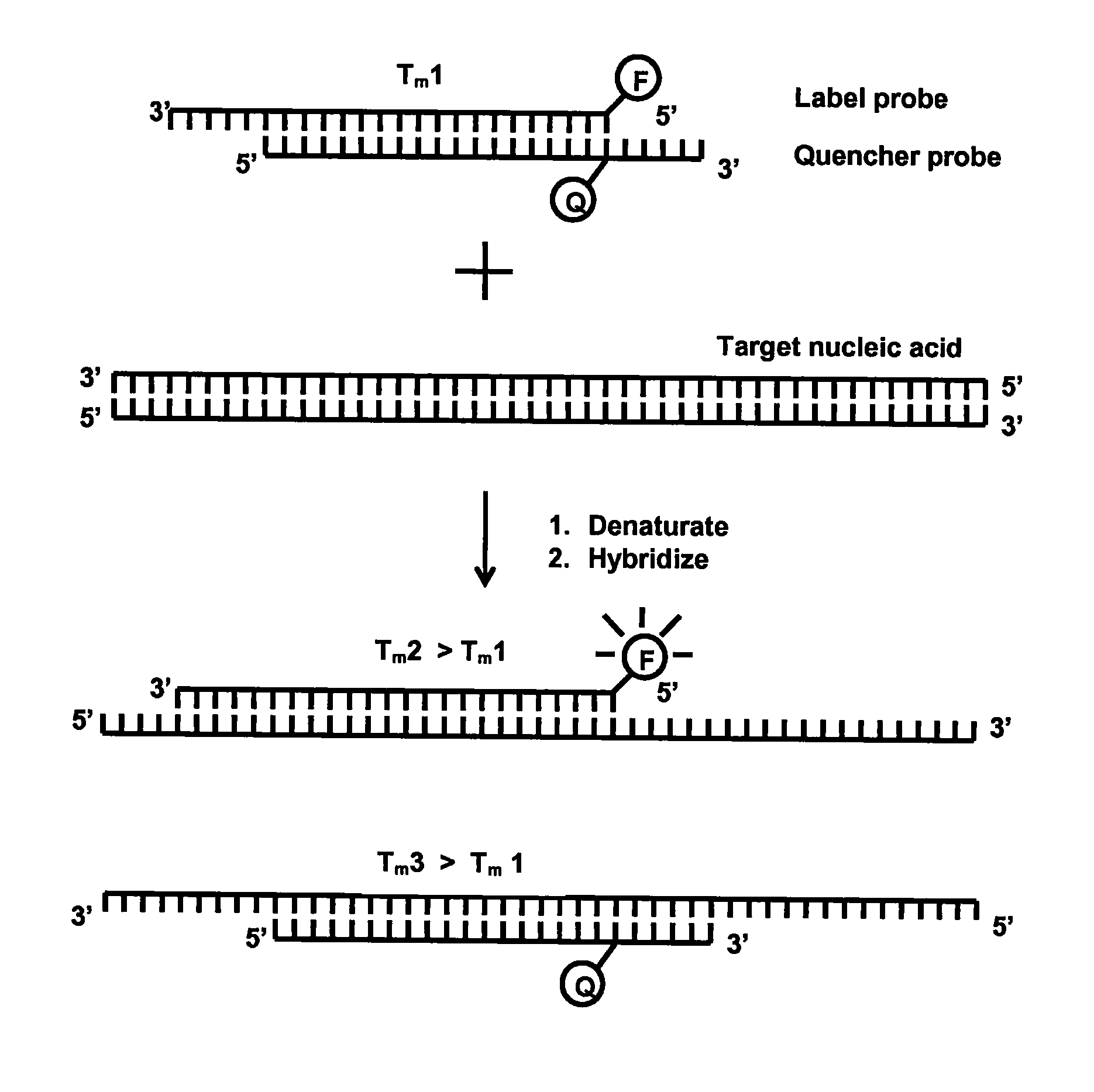
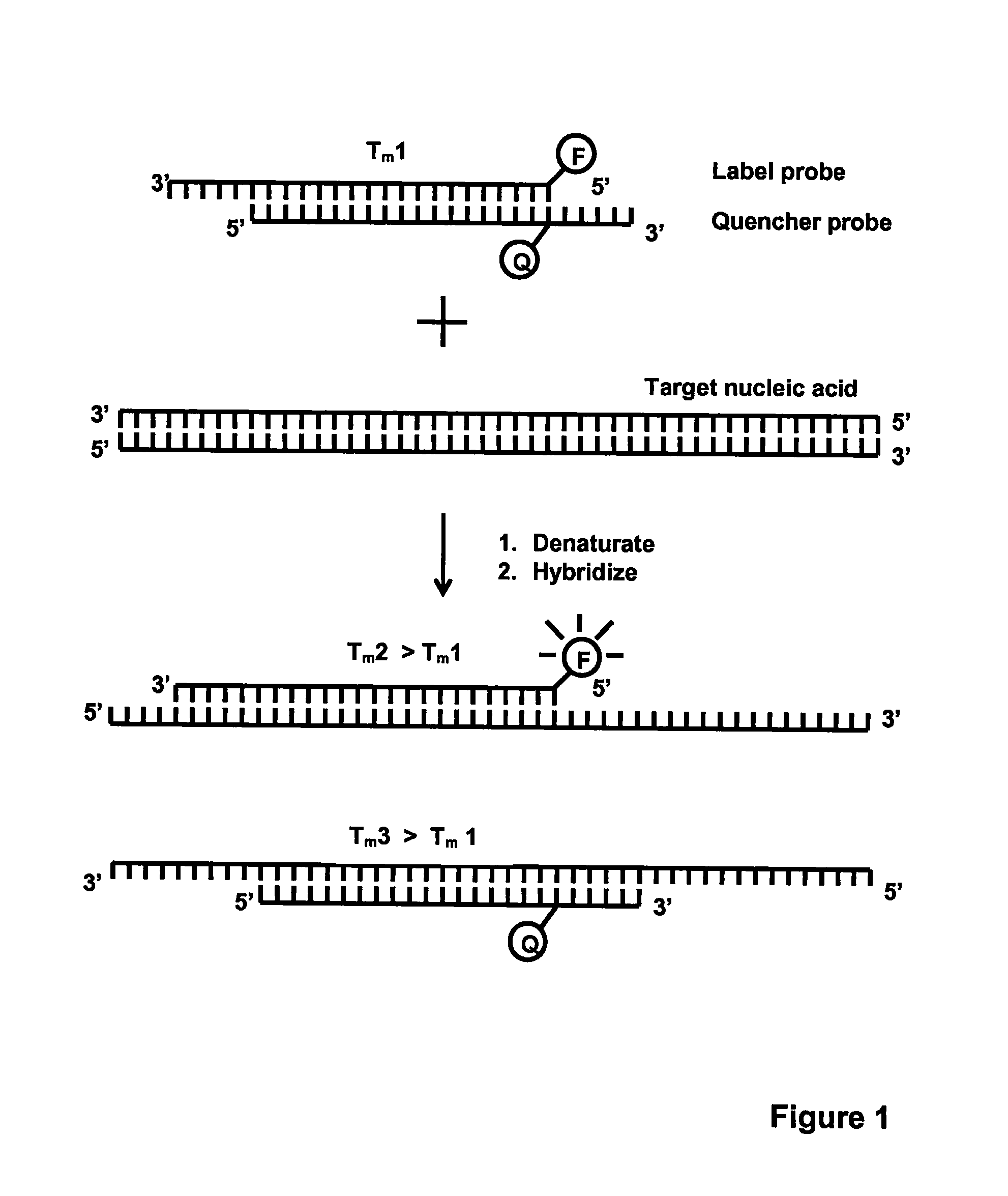
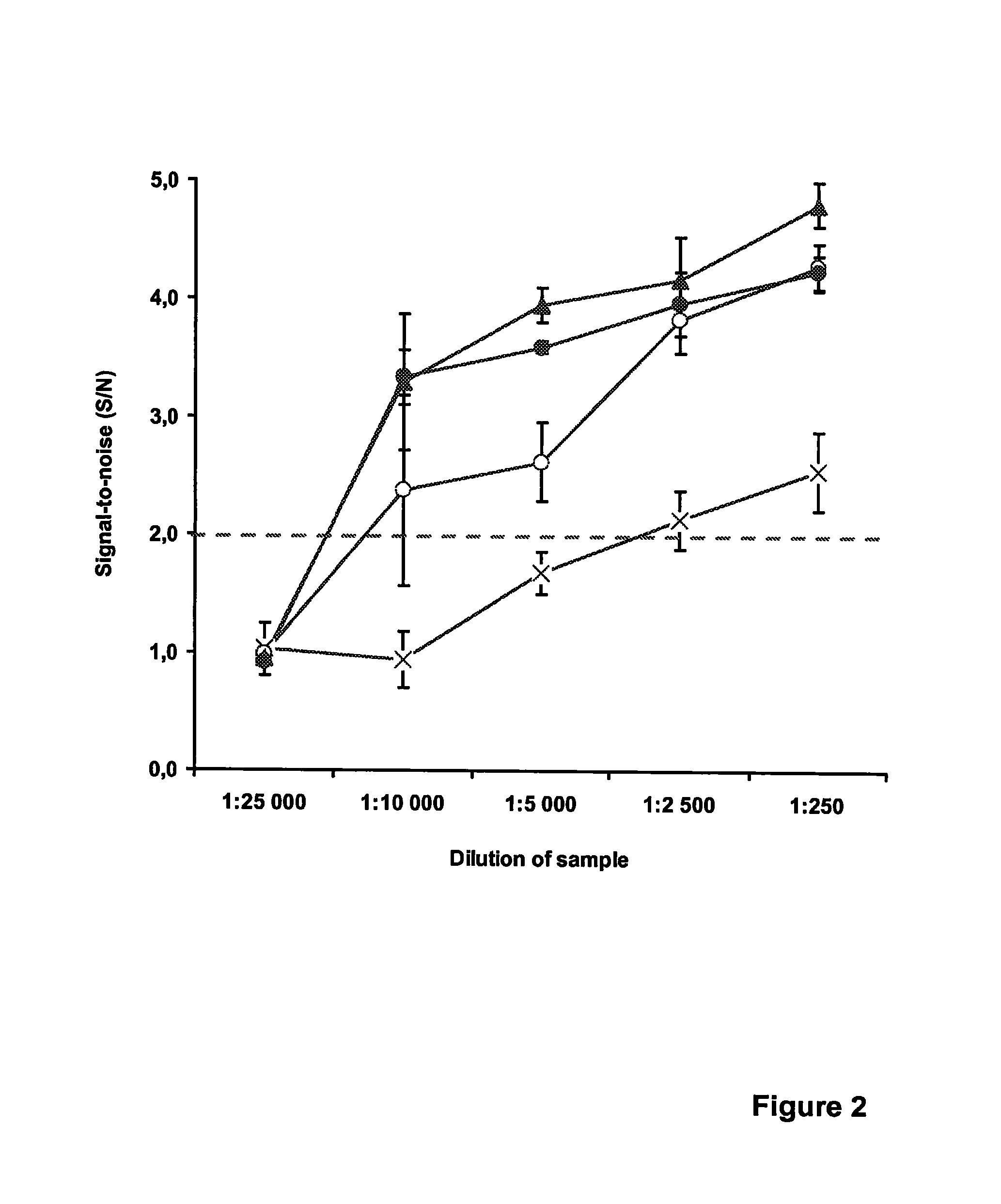
Method for detecting nucleic acids which employs a double-stranded oligonucleotide probe containing i) a first probe including a first label moiety, and ii) a second probe partially complementary with the first probe and including a second label moiety capable of interacting with the first moiety when brought in close proximity with each other, the second moiety being a quencher or acceptor of emission of the first moiety. The first or second probe includes a sequence complementary to that of a target nucleotide, and the second or first probe, respectively, includes a sequence complementary to a complement of the target nucleotide sequence of the nucleic acid to be detected. Oligonucleotides for determining Chlamydia trachomatis are also disclosed.
Owner:ABACUS DIAGNOSTICA OY
Nutritional digestive juice for chlamydia neutralization
InactiveCN101565728AAvoid difficultiesAvoid overgrowthMicrobiological testing/measurementPatient needUrethritis
The invention relates to a Nutritional digestive juice for chlamydia neutralization, in particular to a prescription of a cell culture medium used when a urethritis patient needs the examination of the chlamydia cell culture after using a large amount of clinical antibiotics and a preparation method of the cell culture medium. The genital tract chlamydia trachomatis infection is one of the most common diseases which are sexually transmitted. Chlamydia trachomatis is also regarded as the important cause of the pelvic inflammatory disease and the sequelae (tubal infertility and ectopic pregnancy) thereof. The chlamydia cell culture method is the most sensitive and the most reliable method used for examining the chlamydia. The culture medium can detect out the positive result of the genital tract chlamydia after the large amount of clinical antibiotics is used, thereby having the function of the clinical diagnosis guidance and the clinical treatment and the important meaning of preventing the abuse of the antibacterial drugs.
Owner:曲奕
Rapid fluorescence PCR detection kit for Chlamydia trachomatis
InactiveCN105018574AReduce the risk of contaminationSimple and fast operationMicrobiological testing/measurementNucleic acid detectionProteinase K
The invention belongs to the field of in-vitro nucleic acid detection and aims to provide a rapid detection kit applicable to Chlamydia trachomatis in a clinical sample and used for assisted diagnosis of Chlamydia trachomatis. A rapid fluorescence PCR detection kit for Chlamydia trachomatis comprises a PCR liquid, a negative quality control material, a positive quality control material, a weak positive quality control material, lysate and proteinase K, wherein the PCR liquid contains TaqDNA polymase for hot starting, forward and reverse primers and SYBRGreenI pigment, and the forward and reverse primers are specific primers designed for specific sequence of Chlamydia trachomatis and are capable of specifically amplifying a target DNA sequence, so that the clinical diagnosis purpose is achieved. The kit disclosed by the invention can be used for simply and rapidly detecting the infection of Chlamydia trachomatis in the clinical sample and has the characteristics of high specificity and high sensitivity.
Owner:兰州安康伯乐生物技术有限公司
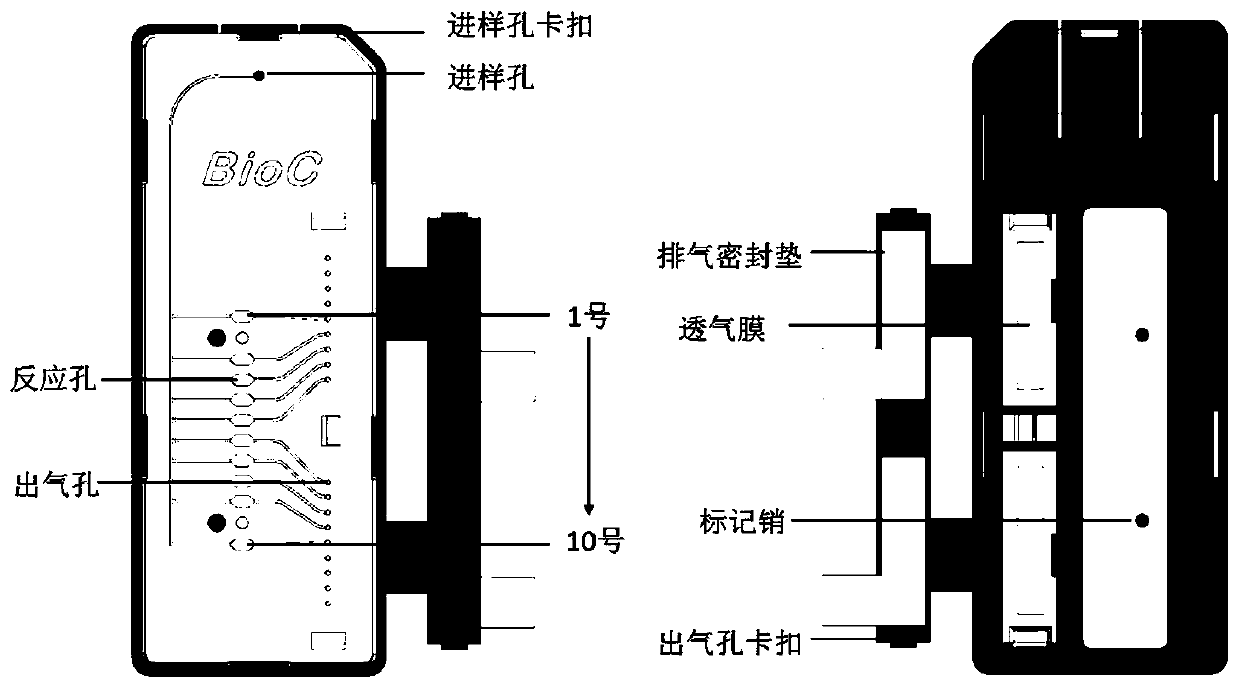
Integrated nucleic acid detection cassette for chlamydia trachomatis
ActiveCN112779248ALower requirements for building standardsAvoid pollutionMicrobiological testing/measurementMicroorganism based processesBiotechnologyNucleic acid detection
The invention discloses an integrated nucleic acid detection cassette for chlamydia trachomatis, which comprises a cassette body with a cassette cover at the top, a lysis cavity, a first cleaning cavity and a second cleaning cavity are sequentially arranged in the cassette body from top to bottom, the lysis cavity is communicated to the cassette cover, and the second cleaning cavity is communicated to a reaction cavity arranged at the bottom of the outer side of the cassette body; plungers with the plunger hole direction perpendicular to the connecting direction are arranged in the pairwise connecting intervals of the cracking cavity, the first cleaning cavity, the second cleaning cavity and the reaction cavity respectively, and hydrophobic liquid sealing materials are arranged in the plunger holes of the plungers; and an amplification reaction solution is arranged in the reaction cavity, a soluble material layer is arranged in the reaction cavity, and a primer probe mixed solution for Aomp gene of chlamydia trachomatis is embedded in the soluble material layer. According to the invention, the chlamydia trachomatis can be rapidly and integrally subjected to PCR amplification and detection under the condition of relatively low cost, and the problems of poor repeatability, time consumption of a detection method, complicated operation and the like in the prior art are solved.
Owner:杭州遂曾生物技术有限公司
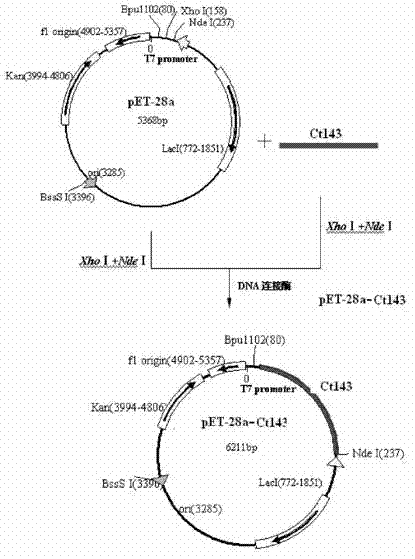
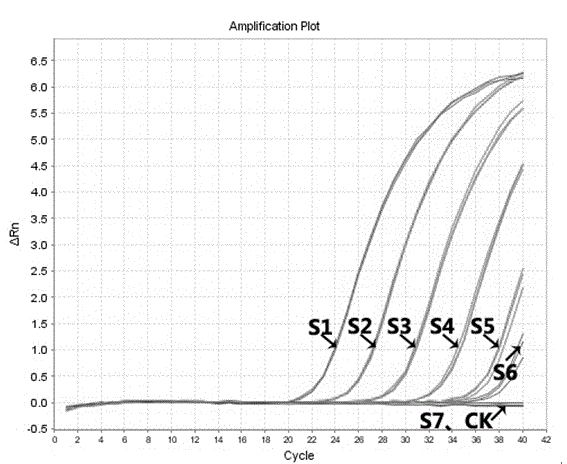
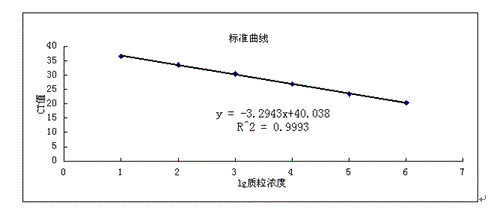
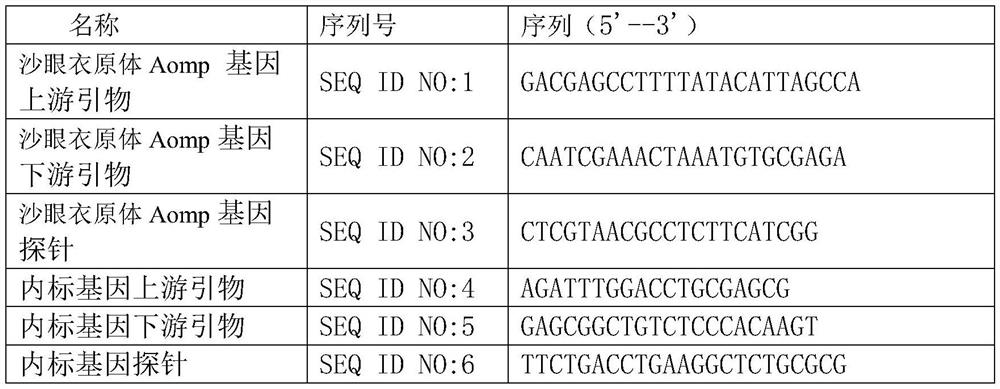
A codon-optimized Chlamydia trachomatis ctl0286 gene and its application
ActiveCN113373163BSenses disorderPeptide/protein ingredientsPharmaceutical drugBiomedical technology
The invention discloses a codon-optimized Chlamydia trachomatis ctl0286 The gene and its application belong to the technical field of biomedicine. The codon-optimized Chlamydia trachomatis ctl0286 Gene, the sequence of which is shown in SEQ ID NO.1. The present invention realizes the in vitro expression of the Chlamydia trachomatis protein CTL0286 that cannot be expressed in the E. coli system through a codon optimization strategy, and the antibody prepared by using the expressed protein can be used for the identification of the CTL0286 protein; as a specific protein of unknown function in Chlamydia trachomatis, the present invention It can provide the basis for functional research and antibody development of CTL0286 protein, and then provide new targets and drugs for clinical treatment and identification of chlamydia.
Owner:NANTONG UNIVERSITY
Assay for detecting Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium
ActiveUS11015228B2Microbiological testing/measurementNucleotideChlamydia trachomatis+Neisseria gonorrhoeae
The invention is directed to methods, kits, and compositions, for amplifying and detecting Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV), and Mycoplasma genitalium (MG) in a sample, which comprises a variety of combinations of forward oligonucleotide primers, reverse oligonucleotide primers, and oligonucleotide probes.
Owner:ABBOTT MOLECULAR INC
Fluorescent quantitative PCR kit for detecting rubella viruses
PendingCN110964855AGuaranteed testingImprove reliabilityMicrobiological testing/measurementDNA/RNA fragmentationTryptophanGenotype
The invention discloses a fluorescent quantitative PCR kit for detecting rubella viruses. The kit comprises a PCR reaction solution, an internal standard, a positive quality control material and a negative quality control material, wherein the PCR reaction solution comprises an upstream primer, a downstream primer and a fluorescent probe which are used for detecting rubella virus RNA, and the internal standard material and the positive quality control material are respectively RNA pseudovirus containing chlamydia trachomatis tryptophan synthetase beta subunit encoding gene and RNA pseudoviruscontaining rubella virus specific conservative gene segment. The kit provided by the invention can detect all genotypes of rubella viruses published in rubella epidemiology in China, adopts pseudoviruses as internal standards, can truly and effectively carry out quality control on possible false negative detection results, and greatly improves the detection sensitivity, accuracy and stability.
Owner:WUHAN BIOTECH GENE ENG
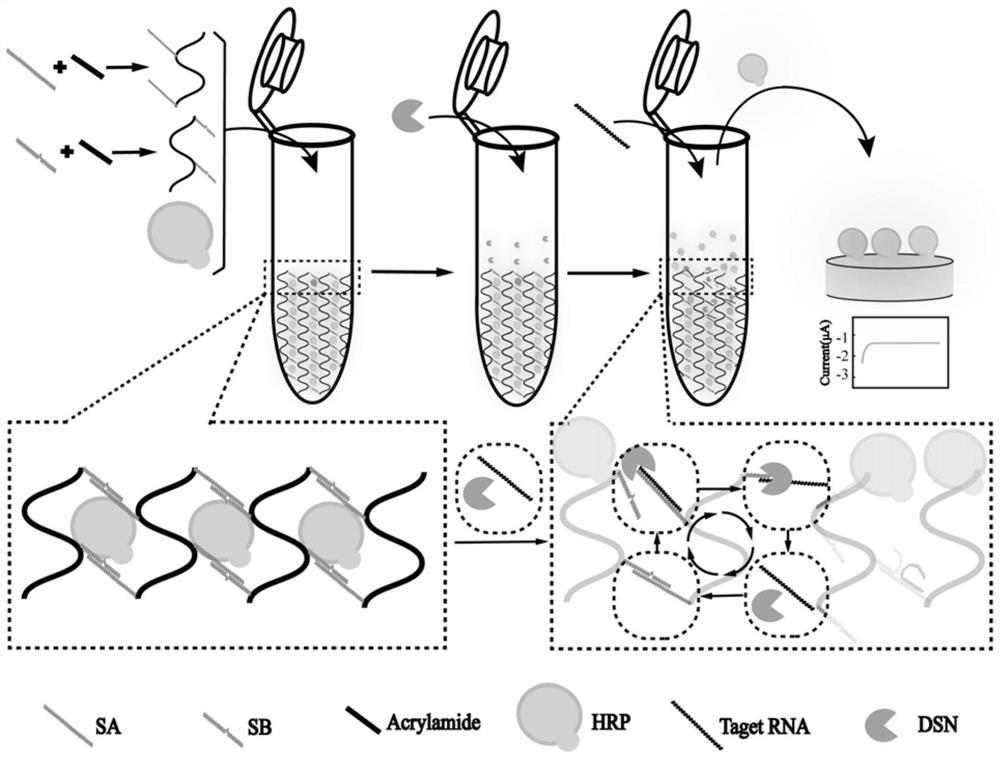
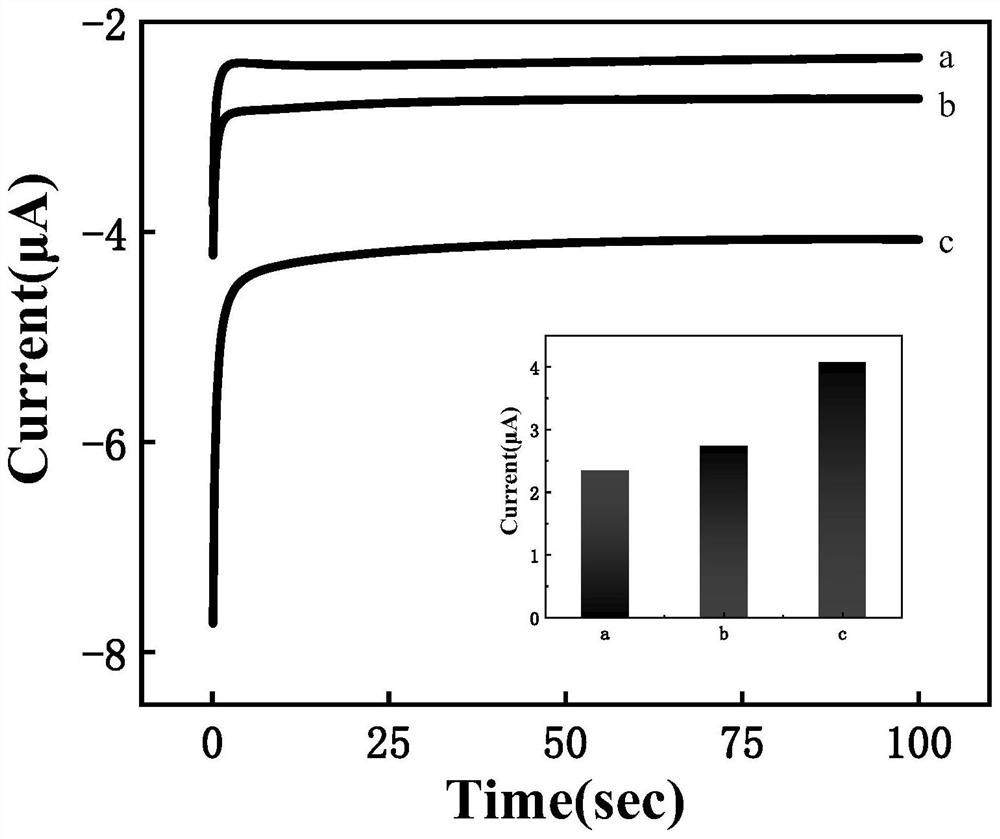
Preparation method of controllable release electrochemical DNA hydrogel composite material based on double-strand specific nuclease assistance
PendingCN113702462AResponsiveIncrease reaction rateMaterial analysis by electric/magnetic meansAgainst vector-borne diseasesA-DNANuclease
The invention discloses a preparation method of a controllable release electrochemical DNA hydrogel composite material based on double-strand specific nuclease assistance. The method is characterized by comprising the following steps of designing base sequences of a DNA chain A (SA) and a DNA chain B (SB) in the DNA hydrogel. Under the assistance of double-strand specific nuclease (DSN), more obvious response can be generated to the stimulation of a small amount of chlamydia trachomatis 16S rRNA (target RNA); in most types of DNA hydrogels, each separation of a DNA probe requires one target chain to hybridize thereto; and when the quantity of target rRNA is less, the target rRNAcannot generate effective stimulation on the DNA hydrogel. According to the method, DSN is successfully introduced into the DNA hydrogel, and the controlled-release DNA hydrogel which can be repeatedly split by a small amount of target objects is obtained, so that the controlled-release DNA hydrogel has higher reaction rate and sensitivity.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV
Chimeric momp antigen, method and use
ActiveCN103068837BEasy to purifyImprove stabilityAntibacterial agentsAntibody mimetics/scaffoldsAntigenBioinformatics
Owner:SPIXIA BIOTECH
Chlamydia trachomatis antigen detection method and kit
InactiveCN111983224ALow skill level requiredHighlight substantiveBiological testingAntigenColloidal au
The invention provides a chlamydia trachomatis antigen detection method, which comprises the steps of: loading a colloidal gold labeled chlamydia trachomatis antibody on a solid nitrocellulose membrane, and coating the solid nitrocellulose membrane with a detection line prepared from an anti-chlamydia lipopolysaccharide monoclonal antibody-II and a control line prepared from an anti-mouse, anti-rabbit and anti-sheep IgG secondary antibody; mixing secreta of a human body with a 0.2 mol / L HCl solution; and putting the sample solution into a sample treatment tube containing a 0.2 mol / L NaOH solution, and dropwise adding the sample solution onto a solid nitrocellulose membrane, wherein when the solid nitrocellulose membrane displays a detection line and a control line at the same time, the secretion of the human body contains chlamydia trachomatis antigen, and the dispersing agent is prepared from a 0.2 mol / L NaOH solution and a 0.2 mol / L HCl solution which have the same volume. The chlamydia trachomatis antigen detection method provided by the invention is a one-step method, the specific high specificity rarely causes the problems of false negative or false positive and the like puzzling clinic, and the chlamydia trachomatis antigen detection method has the advantages of high sensitivity, simple operation and short time consumption.
Owner:郑州迪安医学检验所有限公司
Use of peptidomimetic compounds in the preparation of drugs for inhibiting intracellular growth of Chlamydia trachomatis
ActiveCN109675014BReduce in quantityReduce areaAntibacterial agentsSenses disorderPharmaceutical drugMicrobiology
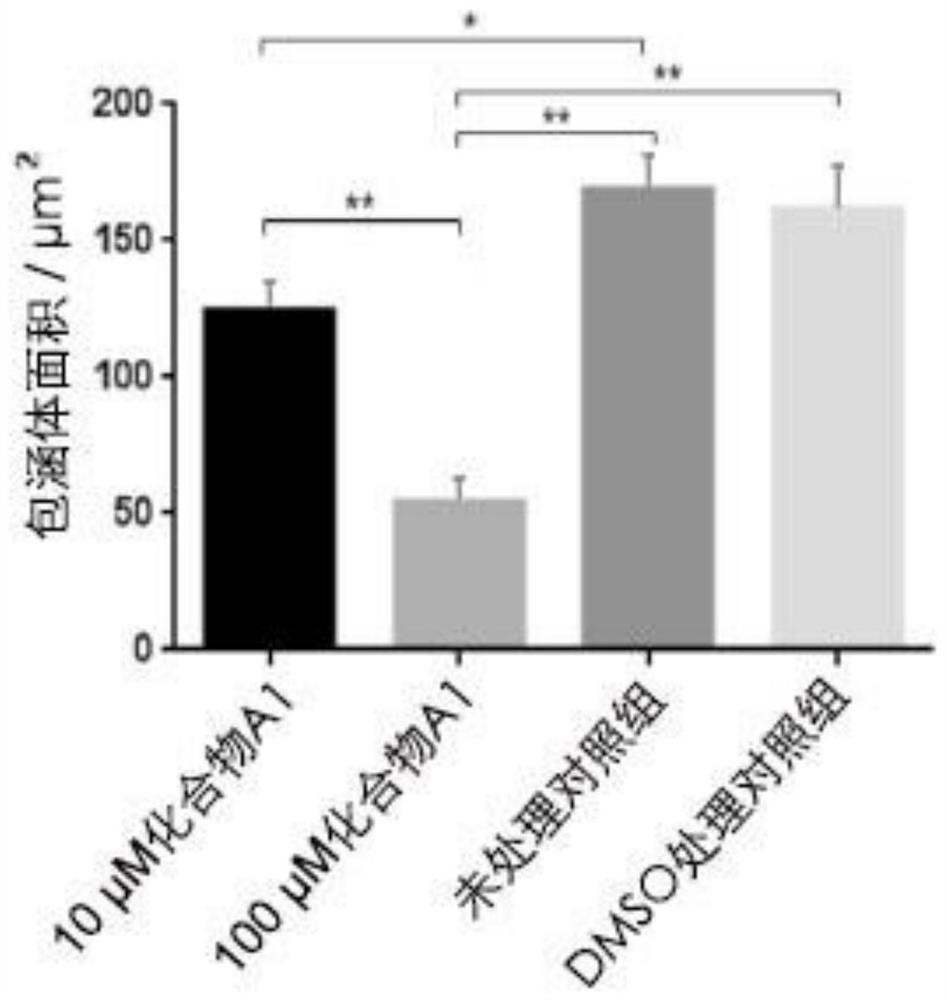
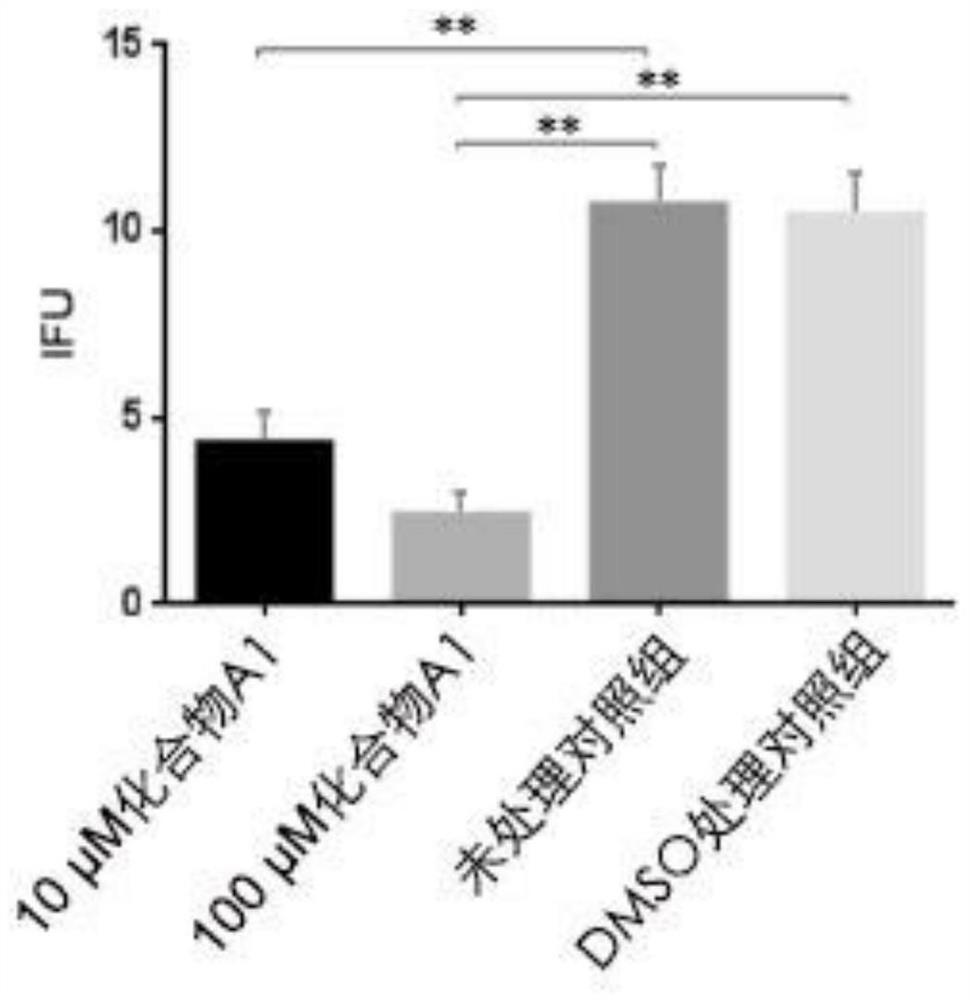
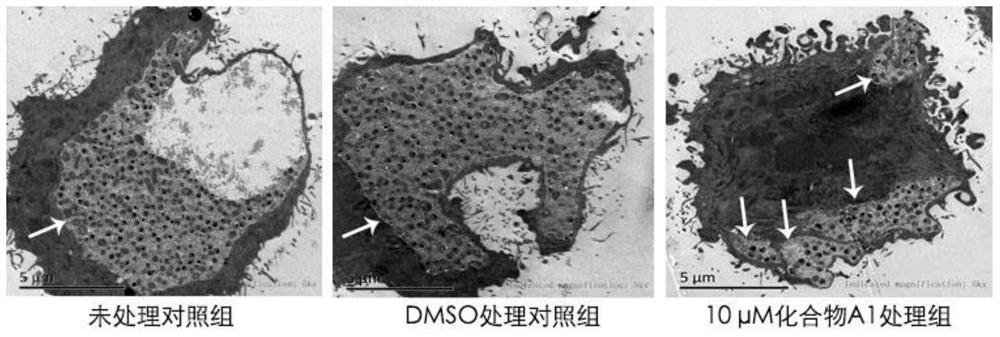
The invention provides application of a mimetic peptide compound in preparing a drug for inhibiting intracellular growth of chlamydia trachomatis. The mimetic peptide compound can directly act on HtrA(High Temperature Requirement A) protein of the chlamydia trachomatis and is capable of inhibiting intracellular growth of the chlamydia trachomatis in the level of 10 muM, the quantity and the areaof chlamydia trachomatis inclusion bodies are reduced, and a new path is provided for treating chlamydia trachomatis infection.
Owner:CENT SOUTH UNIV
Staining kit and staining method, use thereof
InactiveCN1167807CProminent malignant featuresGood malignant featuresMicrobiological testing/measurementDiseaseStaining
The present invention relates to a staining kit and its preparation method, staining method and its application in several detection items for detecting canceration cell, red blood cell, campylobacteriosis, vaginal cleaning degree, estrogen level, garlandosus, trichomoniasis, mycosis, diplococcus gonorrhoeae and chlamydi trachomatis on a cervical smear. Said invented kit is formed from fixatino fluid, staining solution and differentiatino solution. Its staining method includes the steps of fixation, staining, differentiation and water-washing. Its film-making speed is quick, only has need of two main, and its detection accuracy rate is high, can be up to 98%.
Owner:裴芳君
Polypeptide with binding affinity to chlamydia trachomatis MOMP and application of polypeptide
The invention relates to a polypeptide specifically binding to a main outer membrane protein of chlamydia trachomatis and an application of the polypeptide. The polypeptide with binding affinity to the main outer membrane protein of chlamydia trachomatis is disclosed for the first time. The invention also provides an application of the polypeptide in diagnosis and detection, and a diagnosis or treatment application of the polypeptide as a targeting vector in drugs or molecular targeting reagents.
Owner:WENZHOU MEDICAL UNIV
Detecting and parting method of chlamydia trachomatic PCR RLB and used primer, probe
InactiveCN1724690AAccurate typingMicrobiological testing/measurementStrain specificityOligonucleotide primers
The invention discloses a subtype method to testing Chlamydia trachomatis PCR-RLB, and the primer and probe. Three pair oligonucleotide primer, three strain specificity probes, 15 specific type specificity probes are designed aimed at the DNA sequence of kinds of Chlamydia trachomatis. It supplies a method to testing CT infection while CT diagnosing genitourinary tract, taking subtype accurately to CT, and diagnosing mixed infection. It lays a foundation for CT pathogenesis research.
Owner:深圳市慢性病防治院
Secreted chlamydia polypeptides, polynucleotides coding therefor, therapeutic and diagnostic uses thereof
InactiveCN1856505AAtherosclerosisInhibition of secretionAntibacterial agentsBacteriaPreventive vaccinationBacterial strain
The present invention relates to secreted Chlamydia polypeptides, which may be expressed by a Gram-negative bacterial strain and secreted by the type III secretion pathway of said bacterial strain. The present invention also relates to polynucleotides coding for these polypeptides, as well as to the therapeutic and vaccination uses of these secreted Chlamydia polypeptides.
Owner:INST PASTEUR +1
Triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum/ chlamydia trachomatis
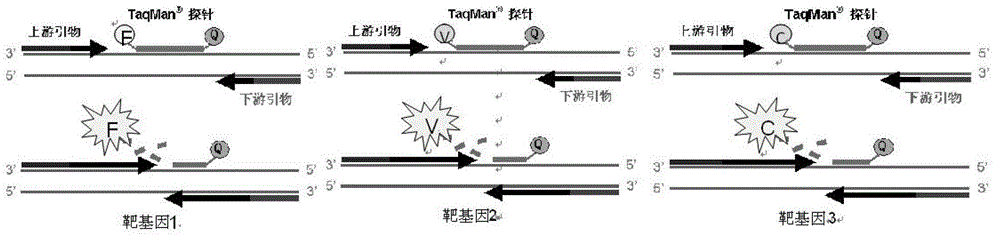
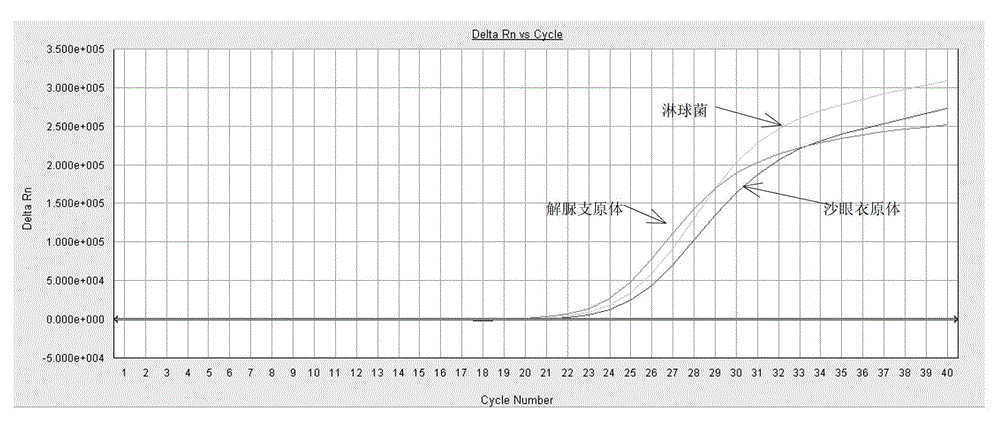
ActiveCN103409508BSimple and fast operationAvoid pollutionMicrobiological testing/measurementFluorescence/phosphorescenceNucleic acid detectionUreaplasma urealyticum
The invention discloses a triple nucleic acid detection kit for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a triple reaction liquid for neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that in the prior art, neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis is poor in specificity, lower in sensitivity and the like, effectively prevents pollution, has the advantages of high sensitivity, good specificity, strong repeatability, quick and objective detection result and the like, and has good application prospect for detecting neisseria gonorrhoeae / ureaplasma urealyticum / chlamydia trachomatis.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com