Chlamydia trachomatis recombinant protein and preparation method thereof
A technology for Chlamydia trachomatis and protein, which is applied in the fields of vaccine development, genetic engineering technology and diagnostic reagents, can solve problems such as reducing detection specificity, and achieve the effect of high sensitivity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
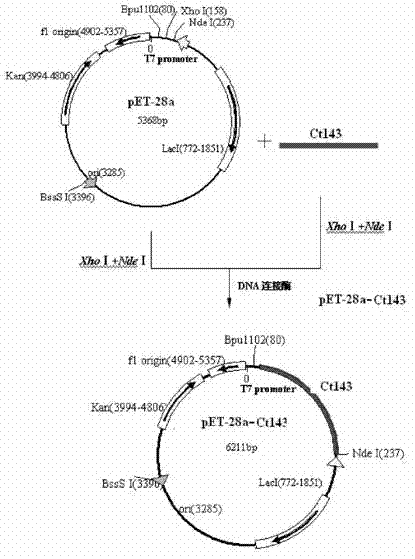
[0024] The preparation of embodiment 1 Chlamydia trachomatis recombinant protein
[0025] 1.1 Screening of Chlamydia trachomatis protein antigen epitope and target gene cloning
[0026] Obtain the full-length sequence of the Ct143 gene of D standard strain (GenBank: NC 000117.1) from GenBank (http: / / www.ncbi.nlm.nih.gov / Entrez), design a pair of specific primers with this DNA sequence as a template, and Add NdeI and XhoI restriction sites and protective bases in the upstream and downstream respectively, and the designed specific primer sequence is as follows: F1: ATCG CATATG ATGAAGAAACCAGTATTTACAGGGGG 41.67%Tm=64.64R1: GATC CTCGAG TTAATCTGCCTCCTTATAAGAAG42.42%Tm=63.49
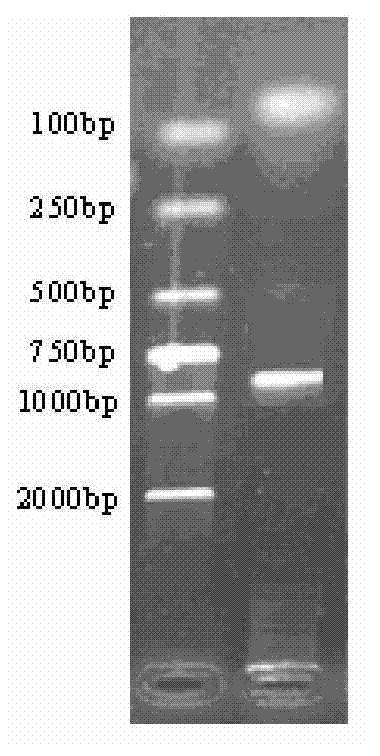
[0027] Using the genome of Chlamydia trachomatis D standard strain D / UW.3 / CX (purchased from China Institute for the Control of Pharmaceutical and Biological Products) as a template, PCR amplification was performed with primers F1 and R1, and the Ct143 fragment was amplified;
[0028] The 50μl PCR amplif...
Embodiment 2
[0049] Example 2 Preparation and Performance Test of Chlamydia trachomatis IgM Antibody Colloidal Gold Detection Kit
[0050] 2.1 Preparation of Chlamydia trachomatis IgM Antibody Antibody Colloidal Gold Detection Kit
[0051] The recombinant Ct protein prepared above was used as the labeled antigen of the kit, and the CT-IgM antibody was detected by the colloidal gold method. The kit was developed and used as follows:
[0052] (1) Principle of the kit
[0053] According to the principle of immune capture method, the invention uses mouse anti-human IgM (μ chain) monoclonal antibody and rabbit anti-chlamydia trachomatis antibody to coat nitrocellulose membrane, and colloidal gold marks recombinant chlamydia trachomatis (Ct) antigen as a tracer. When using, add the serum to be tested. If the sample contains CT-IgM antibody, it can combine with colloidal gold-labeled Ct antigen to form a complex. The complex is captured at the coated mouse anti-human IgM antibody and reacts wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com