Intermediate of synthetic imipenem medicine as well as preparation method and application thereof
A technology of penems and intermediates, which is applied to the preparation of sulfonamides, chemical instruments and methods, and compounds of elements in group 4/14 of the periodic table, etc., which can solve problems such as high cost, poor availability, and complicated preparation process , to achieve the effects of low cost, easy preparation and good product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of (R)-4-methyl-N-(1-phenylethyl)benzenesulfonamide
[0044]
[0045]Add 121g (1mol) (R)-methylbenzylamine, 500ml of dichloromethane and 101g (1mol) of triethylamine into the there-necked flask, stir and cool down to 0°C, add 190g (1mol) of TSCl dropwise, and keep the temperature constant Above 10°C, stir the reaction at 0-10°C for 30 minutes. 300 ml of 1N hydrochloric acid, 300 ml of 5% sodium bicarbonate and 300 ml of water were added to wash the organic phase. A large number of crystals appeared after rotary evaporation and concentration, and then 300ml of methanol was added to the concentrate, stirred and heated to dissolve most of them. Stir intermittently and cool down to 0°C, and put in the freezer (or refrigerator) at -15°C for 24 hours. Filter, wash with a small amount of methanol, and dry in vacuo. 240 g (87% yield) of (R)-4-methyl-N-(1-phenylethyl)benzenesulfonamide were obtained.
[0046] Mp 97~99℃,
[0047] [α]=+72° (c=0.4, EtOH)
[...
Embodiment 2
[0050] 2) Preparation of 2-bromo-N-((R)-1-phenylethyl)-N-p-toluenesulfonyl propionamide
[0051]
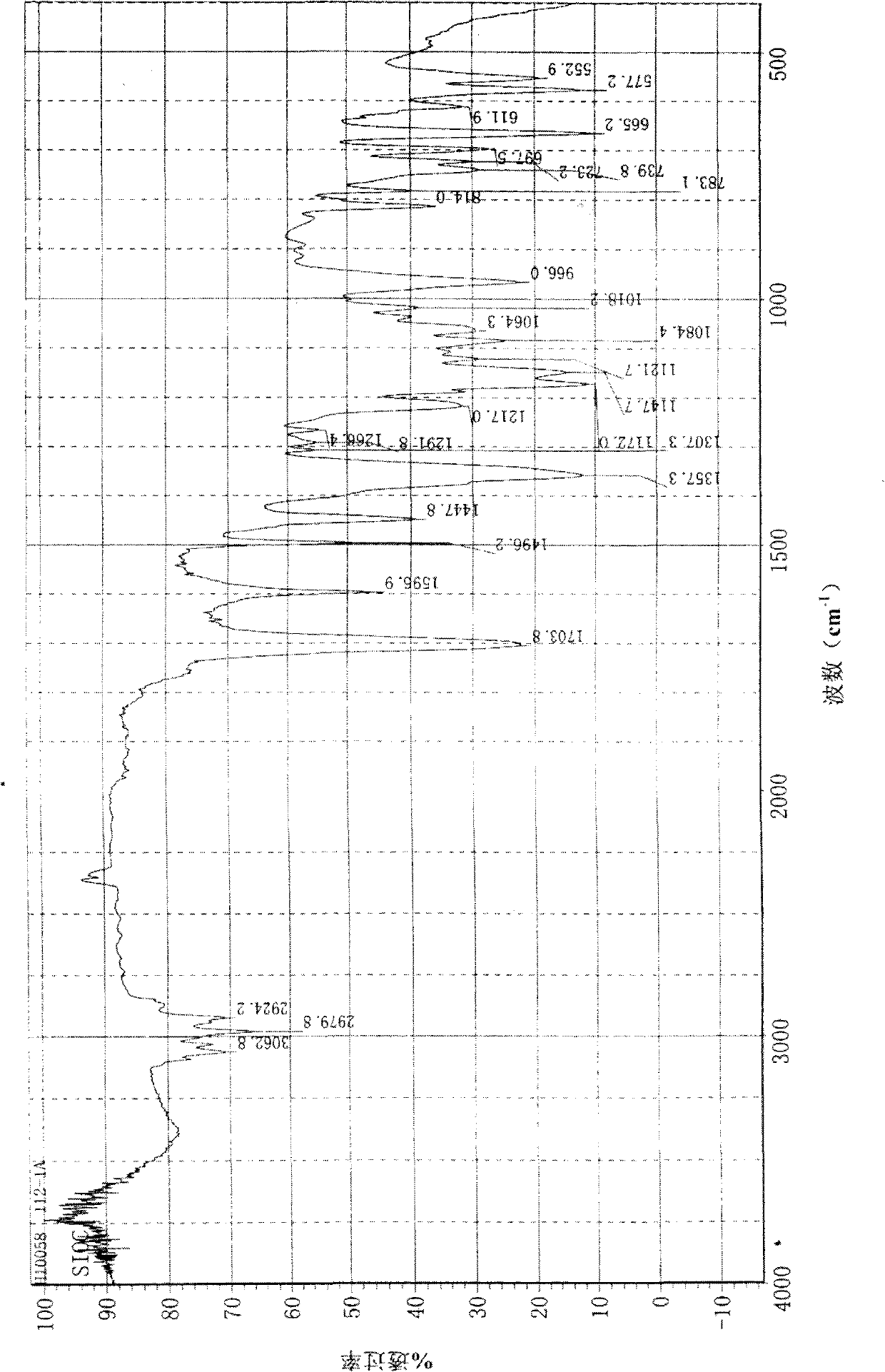
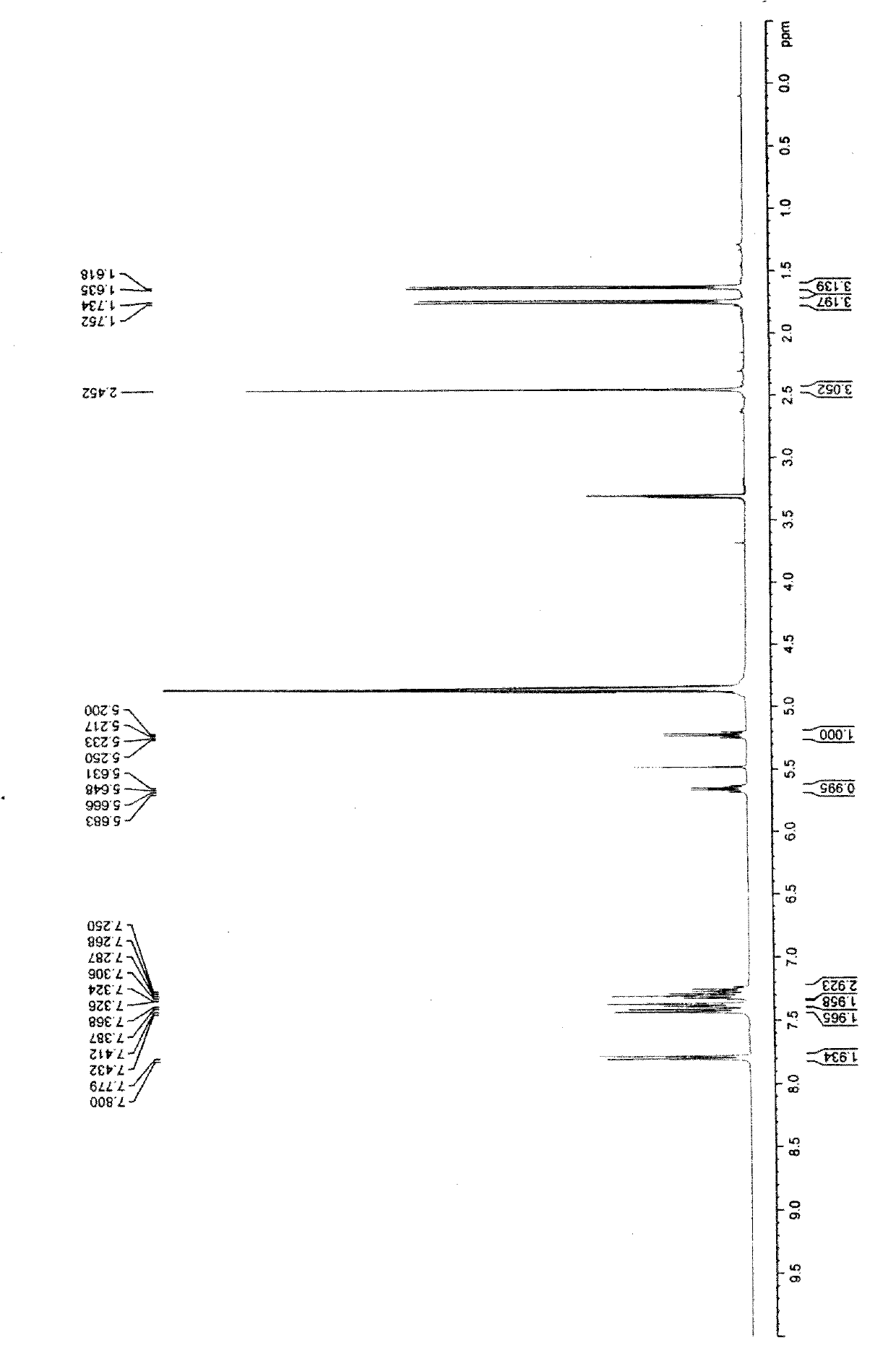
[0052] In the there-necked flask, add 50g (0.182mol) (R)-4-methyl-N-(1-phenylethyl) benzenesulfonamide, 200ml of dichloromethane and 31.2g (0.3mol) of triethylamine and stir , keeping the temperature at 5-10°C, 58.8g (0.273mol) of 2-bromopropionyl bromide was added dropwise, stirred and reacted at 5-10°C for 30 minutes, and then reacted at room temperature for 17h. The organic layer was washed with saturated sodium bicarbonate solution (150ml) and brine (100ml), dried over anhydrous magnesium sulfate, and concentrated to obtain 60.3g (yield 81%) of 2-bromo-N-((R)- 1-phenylethyl)-N-p-toluenesulfonylpropionamide. The infrared spectrum and nuclear magnetic spectrum of the product are as follows figure 1 and figure 2 shown.
[0053] IR (KBr, cm -1 ): 3062, 2979, 2924, 1703, 1596, 1496, 1447, 1357, 1147, 1121, 966.
[0054] 1 H-NMR (200MHz, CDCl3): 1.58 (3H, m), 1.97 (3H, m...
Embodiment 3
[0056] 3) Preparation of 2-bromo-N-(4-chlorobenzenesulfonyl)-N-((R)-1-m-tolylethyl)propionamide
[0057]
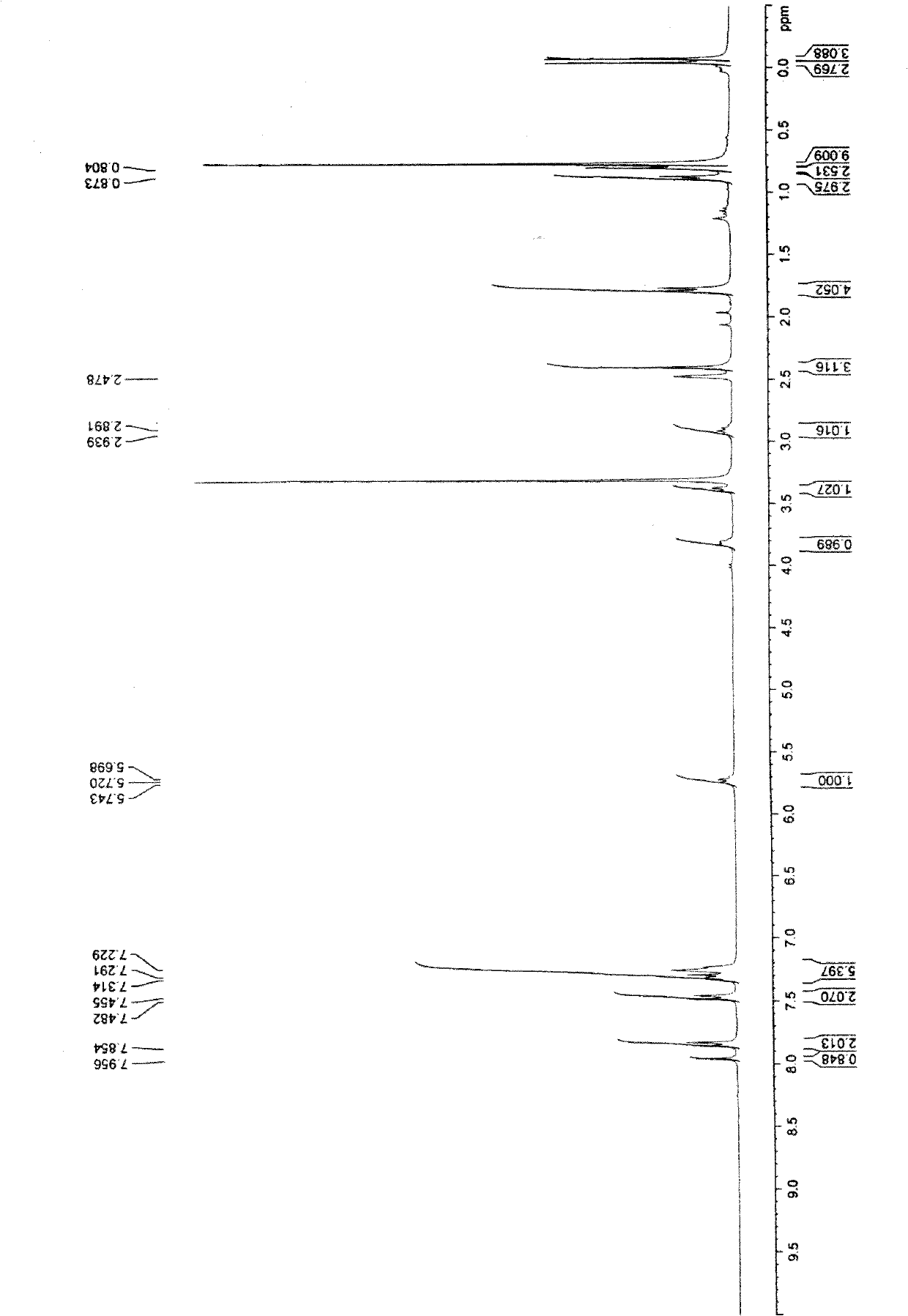
[0058] The same as the operation process of Example 1 and Example 2, the difference is to use 13.5g (0.10mol) of 4-chlorobenzenesulfonyl chloride and 22.1g (0.105mol) of R-1-m-tolylethylamine to obtain white powder 34.6 g (yield 78.2%) 2-bromo-N-(4-chlorobenzenesulfonyl)-N-((R)-1-m-tolylethyl)propanamide.
[0059] Mp: 112~113℃
[0060] 1 H-NMR (200MHz, CDCl3): 1.58 (3H, m), 1.97 (3H, d), 2.35 (3H, s), 4.55 (1H, dd), 5.06 (1H, dd), 6.88-6.93 (3H, m), 7.09(1H,m), 7.61(2H,d), 7.74(2H,d)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com