Lipophilic compound conjugate of cell penetrating peptide and its application in antibiosis
A technology for penetrating peptides and compounds, applied in the direction of antibacterial drugs, antifungal agents, preparation methods of peptides, etc. enhancement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
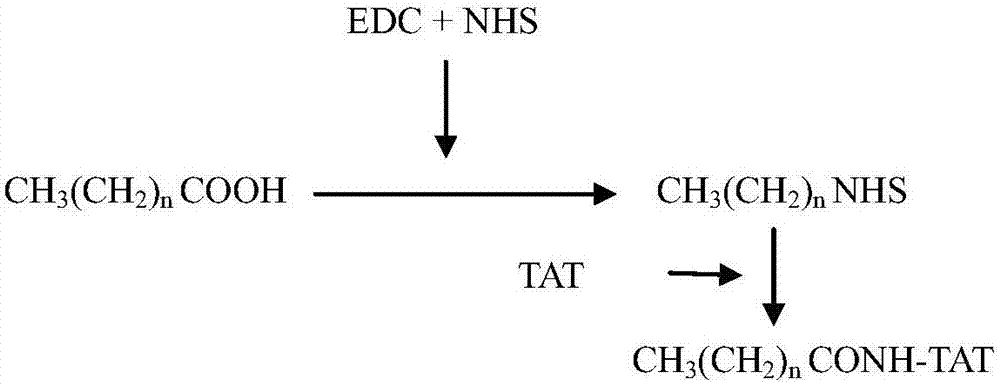
[0083] Embodiment 1, the preparation of lipopeptide
[0084] In this example, six lipopeptides named C4-TAT, C8-TAT, C12-TAT, C14-TAT, C16-TAT and C20-TAT were prepared, all of which were penetrated by cells named TAT Peptide (amino acid sequence is YGRKKRRQRRR ie SEQ ID No. 1), which is formed by linking lipophilic compound connected to the amino terminus of the cell penetrating peptide.
[0085] The structural formula of C4-TAT is: C4-YGRKKRRQRRR, wherein C4 is butyric acid;
[0086] The structural formula of C8-TAT is: C8-YGRKKRRQRRR, wherein C8 is octanoic acid;
[0087] The structural formula of C12-TAT is: C12-YGRKKRRQRRR, wherein C12 is lauric acid;
[0088] The structural formula of C14-TAT is: C14-YGRKKRRQRRR, wherein, C14 is myristic acid;
[0089] The structural formula of C16-TAT is: C16-YGRKKRRQRRR, wherein, C16 is palmitic acid;
[0090] The structural formula of C20-TAT is: C20-YGRKKRRQRRR, wherein C20 is arachidic acid;
[0091] YGRKKRRQRRR in the structur...
Embodiment 2
[0095] Embodiment 2, the antibacterial action of lipopeptide
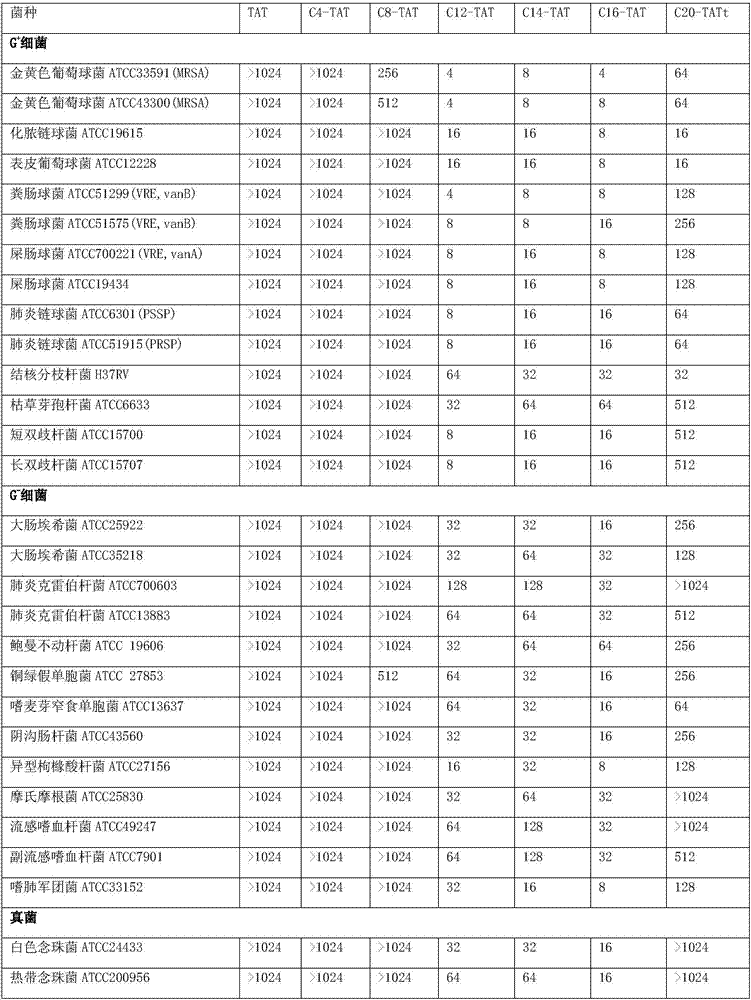
[0096] 1. Determination of minimum inhibitory concentration (MIC)
[0097] Adopt plate double dilution method to carry out drug susceptibility test, measure the minimum inhibitory concentration (MIC) of C4-TAT, C8-TAT, C12-TAT, C14-TAT, C16-TAT and C20-TAT in embodiment 1, specifically The experimental method is as follows: the test bacteria (as shown in Table 2) were cultured with MH broth medium. Drugs (C4-TAT, C8-TAT, C12-TAT, C14-TAT, C16-TAT and C20-TAT in Example 1) are diluted into various required concentrations with MH broth medium twice, and an appropriate amount is added respectively In the petri dish, the MH agar medium is quantitatively injected into the petri dish containing the medicine after melting, and mixed evenly, and various tested microorganisms are inoculated with a multi-point inoculator, (the inoculum size is 10 4 CFU / spot) was placed in a 37°C incubator for 18 hours of constant temperatu...
Embodiment 3
[0120] Embodiment 3, combined application of lipopeptide and antibacterial drug
[0121] 1. Determination of combined use index (FIC) of lipopeptide and antibacterial drugs against drug-resistant bacteria
[0122] Measure respectively the FIC value of the lipopeptide C12-TAT of embodiment 1 and antimicrobial drug combined application to Gram-positive drug-resistant bacterium Staphylococcus aureus ATCC33591 (MRSA), and the C16-TAT of embodiment 1 and antibacterial drug combined application to FIC values of Gram-negative drug-resistant bacteria Pseudomonas aeruginosa ATCC 27853. The method and results are as follows:
[0123] Each antimicrobial drug (as drug A) and lipopeptide (as drug B) respectively took their respective 2MIC as the highest concentration, diluted to 8 concentrations with sterile MH broth medium, respectively, along the horizontal axis of the microwell culture plate, Add 50 μL of MH broth medium containing different concentrations of the two drugs on the ve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com