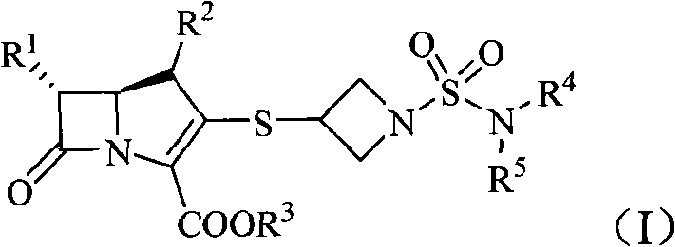
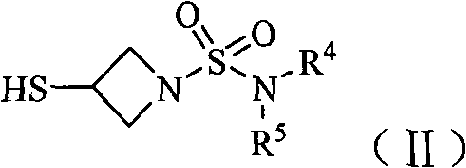
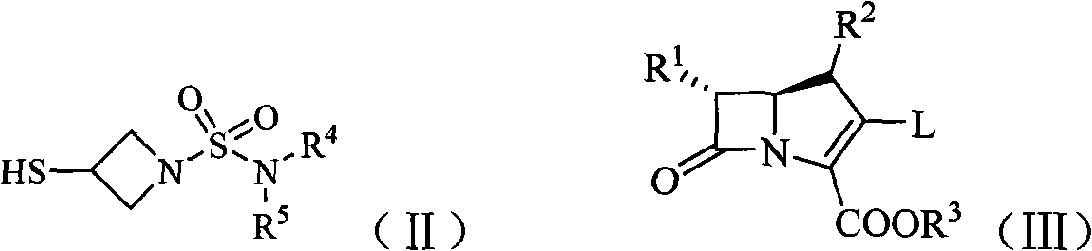Imipenem derivant containing sulfonyl azetidine
An azetidine and aminosulfonyl technology, applied in the field of medicine, can solve the problems of inability to meet clinical needs, increased bacterial resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1 (4R, 5S, 6S)-3-[1-(azetidin-1-yl)sulfonyl-azetidin-3-yl]thio-6-[(1R)- 1-Hydroxyethyl Preparation of base]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound 1)
[0124] Step 1 Preparation of 3-acetylthio-azetidine
[0125] In a dry reaction flask, add 6.9g (0.04mol) of 3-hydroxy-1-tert-butoxycarbonyl-azetidine and 9.2g (0.08mol) of potassium thioacetate, then add 20mL of DMF, stir and heat until dissolved Afterwards, keep the reaction for 12 hours, cool to room temperature, add 100 mL of water to dilute, extract with ethyl acetate, wash the organic phase with water, dry, and concentrate to obtain a yellow oil. Add 20 mL of dichloromethane to the oily substance, then add 10 mL of trifluoroacetic acid (TFA) dropwise at room temperature, stir overnight, TLC detects that the reaction is complete, concentrate, add 50 mL of dichloromethane to the residue, rotary evaporate to dryness, repeat several operations To remove the residual triflu...
Embodiment 2
[0137] Example 2 (4R, 5S, 6S)-3-[1-(aziridin-1-yl)sulfonyl-azetidin-3-yl]thio-6-[(1R)-1- Hydroxyethyl]-4-methyl Base-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (Compound 2)
[0138] 1. With reference to step 1 in Example 1, 3-acetylthio-azetidine was prepared;
[0139] 2. Referring to step 2 in Example 1, 3-mercapto-1-(aziridin-1-yl)sulfonyl-azetidine was obtained;
[0140] 3. Refer to step 3 in Example 1 to prepare (4R, 5S, 6S)-3-[1-(aziridin-1-yl)sulfonyl-azetidin-3-yl]sulfanyl-6 -[(1R)-1-Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate p-nitrobenzyl ester;
[0141] 4. Refer to step 4 in Example 1 to prepare compound 2.
[0142] Molecular formula: C 15 h 21 N 3 o 6 S 2 Molecular weight: 403.47 Mass spectrum (M+1): 404
[0143] Elemental Analysis: Found: C, 44.48%; H, 5.41%; N, 10.23%; S, 16.15%
[0144] Theoretical value: C, 44.65%; H, 5.25%; N, 10.41%; S, 15.89%
[0145] 1 H-NMR (δ / ppm, 300MHz, DMSO): 12.96 (1H, s), 4.96 ...
Embodiment 3
[0146] Example 3 (4R, 5S, 6S)-3-[1-(pyrrolidin-1-yl)sulfonyl-azetidin-3-yl]thio-6-[(1R)-1-hydroxy Ethyl]-4-methyl Preparation of yl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (compound 3)
[0147] 1. With reference to step 1 in Example 1, 3-acetylthio-azetidine was prepared;
[0148] 2. Referring to step 2 in Example 1, 3-mercapto-1-(pyrrolidin-1-yl)sulfonyl-azetidine was obtained;
[0149] 3. Referring to step 3 in Example 1, (4R, 5S, 6S)-3-[1-(pyrrolidin-1-yl)sulfonyl-azetidin-3-yl]thio-6- [(1R)-1-Hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate p-nitrobenzyl ester;
[0150] 4. Refer to step 4 in Example 1 to prepare compound 3.
[0151] Molecular formula: C 17 h 25 N 3 o 6 S 2 Molecular weight: 431.53 Mass spectrum (M+1): 432
[0152] Elemental Analysis: Found: C, 47.16%; H, 6.01%; N, 9.58%; S, 14.98%
[0153] Theoretical value: C, 47.32%; H, 5.84%; N, 9.74%; S, 14.86%
[0154] 1H-NMR (δ / ppm, 300MHz, DMSO): 12.93 (1H, s),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com