Method for synthesizing meropenem intermediate
A synthesis method and technology of meropenem side chain, applied in the direction of organic chemistry, etc., can solve the problems of poor stability, high energy consumption, long working hours, etc., and achieve the effects of improved stability and yield, low yield and long working hours
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
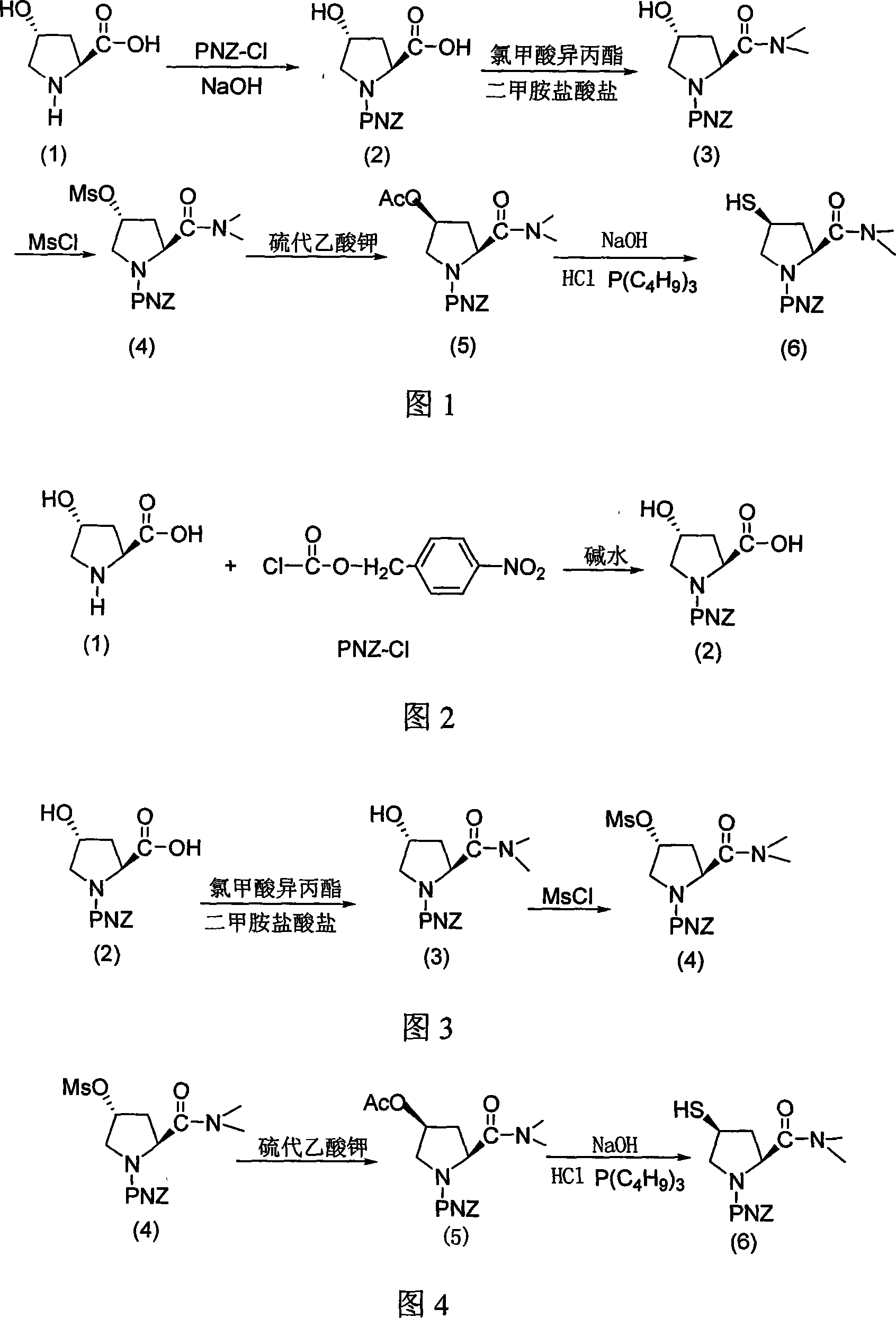
[0024] Example 1: A method for synthesizing the side chain H of meropenem. The chemical name of the side chain H of meropenem is (2s, 4s)-1-(p-nitrobenzyloxycarbonyl)-2-dimethylformylamino-4 -Mercaptopyrrolidine, characterized in that 4R-hydroxyl-L-proline (1) is used as raw material to react with p-nitrobenzyl chloroformate under alkaline conditions to obtain amino-protected 4R-hydroxyl-L-proline Amino acid compound A (2), the intermediate (3) obtained by aminolysis after activating the carboxyl hydroxyl group of compound A (2) with isopropyl chloroformate, and then the intermediate (3) is acylated with methylsulfonyl chloride to obtain compound B (4), compound B (4) was substituted with potassium thioacetate to obtain compound C (5) with inverted 4-hydroxy configuration, and compound C (5) was subjected to alkaline hydrolysis to obtain meropenem side chain H (6).
[0025] A method for synthesizing meropenem side chain H, characterized in that the specific steps are as follow...
Embodiment 2
[0032] Example 2: A method for synthesizing the side chain H of meropenem. The chemical name of the side chain H of meropenem is (2s, 4s)-1-(p-nitrobenzyloxycarbonyl)-2-dimethylformylamino-4 -Mercaptopyrrolidine, characterized in that 4R-hydroxyl-L-proline (1) is used as raw material to react with p-nitrobenzyl chloroformate under alkaline conditions to obtain amino-protected 4R-hydroxyl-L-proline Amino acid compound A
[0033] (2), the intermediate (3) obtained by aminolysis after activation of the carboxyl hydroxyl group of compound A (2) by isopropyl chloroformate, and then the intermediate (3) is acylated with methylsulfonyl chloride to obtain compound B (4), Compound B (4) was substituted with potassium thioacetate to obtain compound C (5) with inverted 4-hydroxyl configuration, and compound C (5) was subjected to alkaline hydrolysis to obtain meropenem side chain H (6).
[0034] A method for synthesizing meropenem side chain H, characterized in that the specific steps a...
Embodiment 3
[0041] Example 3: A method for synthesizing the side chain H of meropenem. The chemical name of the side chain H of meropenem is (2s, 4s)-1-(p-nitrobenzyloxycarbonyl)-2-dimethylformylamino-4 -Mercaptopyrrolidine, characterized in that 4R-hydroxyl-L-proline (1) is used as raw material to react with p-nitrobenzyl chloroformate under alkaline conditions to obtain amino-protected 4R-hydroxyl-L-proline Amino acid compound A (2), the intermediate (3) obtained by aminolysis after activating the carboxyl hydroxyl group of compound A (2) with isopropyl chloroformate, and then the intermediate (3) is acylated with methylsulfonyl chloride to obtain compound B (4), compound B (4) was substituted with potassium thioacetate to obtain compound C (5) with inverted 4-hydroxy configuration, and compound C (5) was subjected to alkaline hydrolysis to obtain meropenem side chain H (6).
[0042] A method for synthesizing meropenem side chain H, characterized in that the specific steps are as follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com