Application of dithiocarbamate derivative to antibacterial field
An aminodithioformate and derivative technology, which is applied in the application field of new metallo-beta-lactamase inhibitors in the field of antibacterial, can solve the problems of unsatisfactory effect of restoring meropenem sensitivity and the like
Active Publication Date: 2017-09-22
ZHENGZHOU UNIV
View PDF3 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In 2015, Yang Kewu's research group at Northwest University reported a series of thioglycolic acid thioester amino acid derivatives, whose IC for metallo-β-lactamase L1 50 The minimum can reach 18nM, but its in vitro activity test pro
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
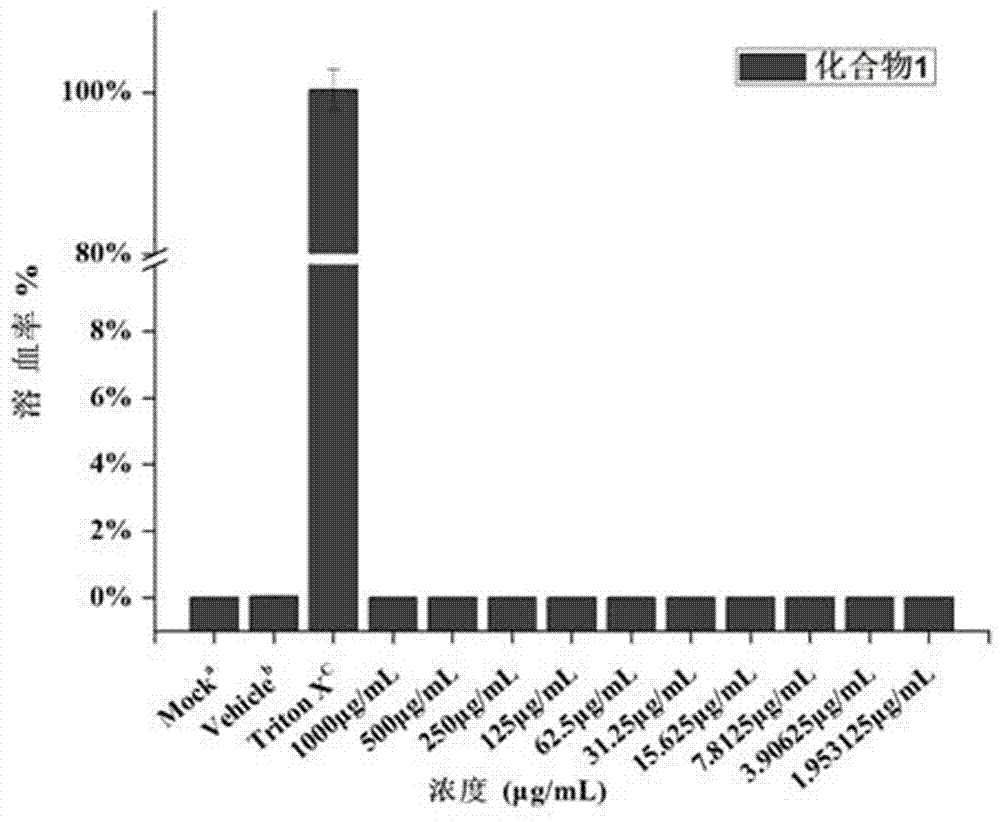
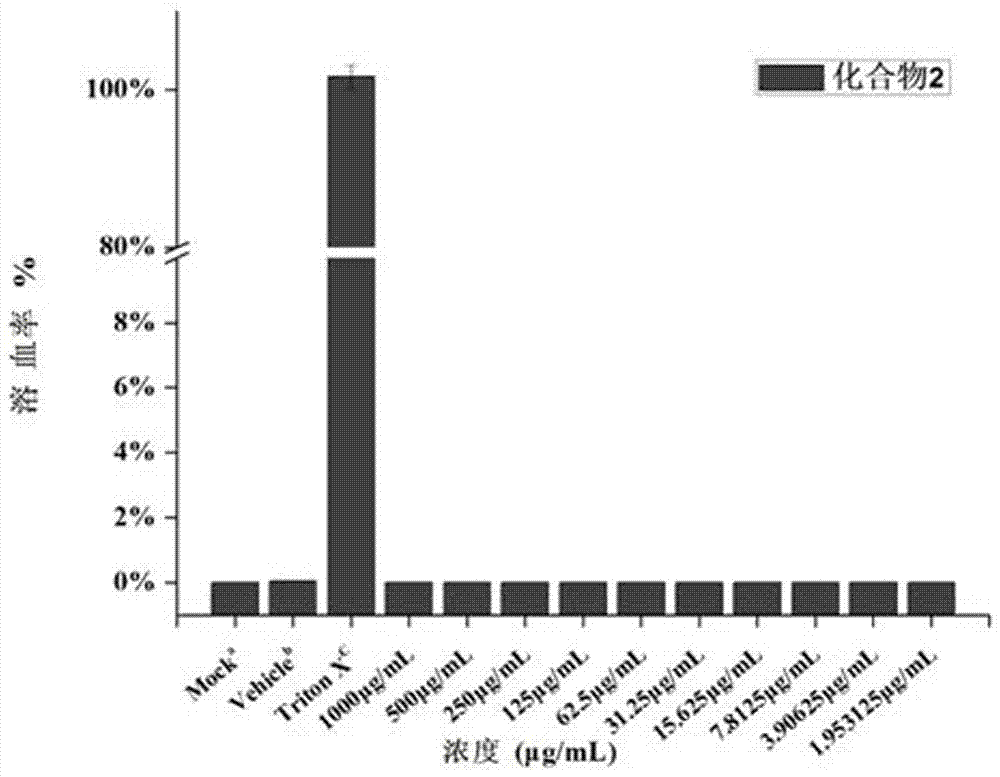
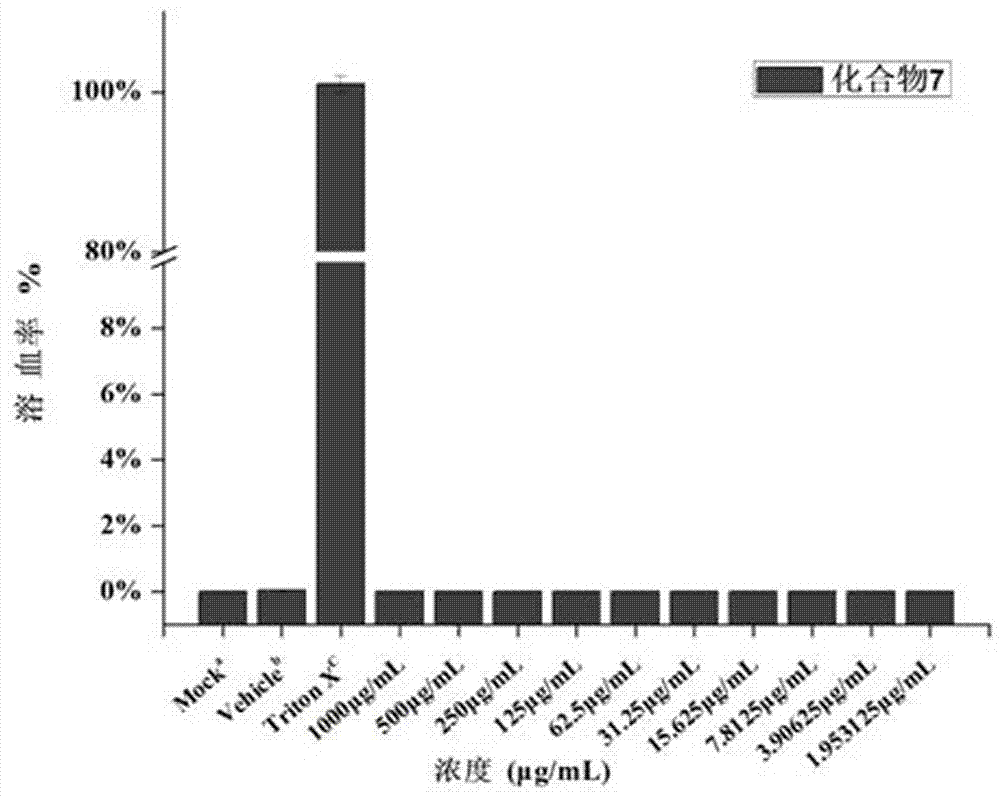
The invention belongs to the field of medicine chemistry, and discloses application of dithiocarbamate derivative to the antibacterial field. By adopting the compound, the sensitivity of enterobacteriaceae bacteria containing metal beta-lactamase to carbapenems antibiotics can be recovered. In vitro antibacterial experimental result proves that the compound is combined with meropenem (MEM), so that the drug sensitivity of the MEM can be improved. For carbapenems-tolerant NDM-1 enzyme-produced escherichia coli, by adopting the compound, an MIC (minimum inhibitory concentration) value of the carbapenems meropenem can be reduced, optimally by about 40960 times. An experiment on the sensitivity of zinc ions preliminarily proves that the zinc ions have interference on recovery of an MEM drug effect by utilizing the compound. The dynamic germicidal efficacy proves that the germicidal efficacy of the compound to MEM also can be greatly promoted under a combination effect. An in vitro red cytotoxicity experiment and a cytotoxicity experiment prove that the toxicity of the compound is lower, and therefore, the compound is relatively safe. The compound can be used as a candidate medicine of a novel metal beta-lactamase inhibitor.
Description
technical field [0001] The invention belongs to the technical field of medicinal chemistry, and relates to the application of amino dithioformate derivatives as novel metallo-beta-lactamase inhibitors in the antibacterial field. Background technique [0002] The trend of Antimicrobial resistance (AMR) has invaded the world like a ghost (Microbiology and Molecular Biology Reviews 2010, 74, 417.). Antibiotic resistance has become a major threat to public health security (Nature Reviews Microbiology 2015, 13, 42.). The reasons for bacterial drug resistance can be attributed to two aspects: bacterial chromosomal mutation and horizontal transmission of drug resistance genes. Among them, the inherent drug resistance of bacteria is the instinct of bacteria themselves. The acquired drug resistance (Nature 2004, 430, 242.) caused by the horizontal transmission of drug resistance genes is an important reason for the dissemination of drug resistance and the emergence of multi-drug re...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/40A61K31/4453A61K31/535A61K31/495A61K31/27A61P31/04
CPCA61K31/27A61K31/40A61K31/4453A61K31/495A61K31/535Y02A50/30
Inventor 张恩秦上尚施秀芳王铭铭王平严妍化永刚白鹏燕崔得运王亚娜刘宏民
Owner ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com