A preparation method of a meropenem intermediate
A technology for meropenem and intermediates, which is applied in the field of preparation of meropenem intermediates, can solve the problems of high reaction risk system, complex reaction process, high cost, etc., and achieve simple and reliable process, high yield and purity, and improved reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
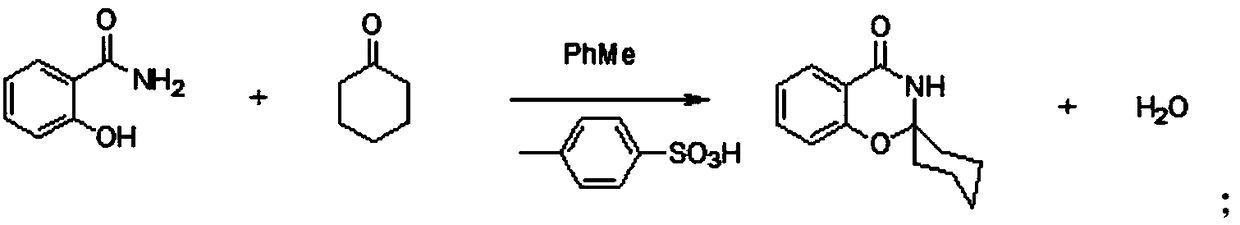
[0026] 1. Synthesis of the first step:
[0027] In the reactor, put toluene first, then drop into 137.14g salicylamide, 1.72g p-toluenesulfonic acid, and 127.58g cyclohexanone, and heat to reflux for 3 hours. After the reflux, cool down to 10-15°C and keep warm for 1 hour, centrifuge to shake the material, wait for drying, then rinse with toluene, and then dry. The finished product (weight after drying) of white crystals was obtained. The HPLC content is above 99%. M1 mother liquor is pumped to atmospheric pressure distillation in the kettle until the residual liquid is basically free of toluene, and then the cyclohexanone content in the distillate is detected by the external standard method, which is applied to the next batch, and the residue in the kettle is disposed of in barrels.
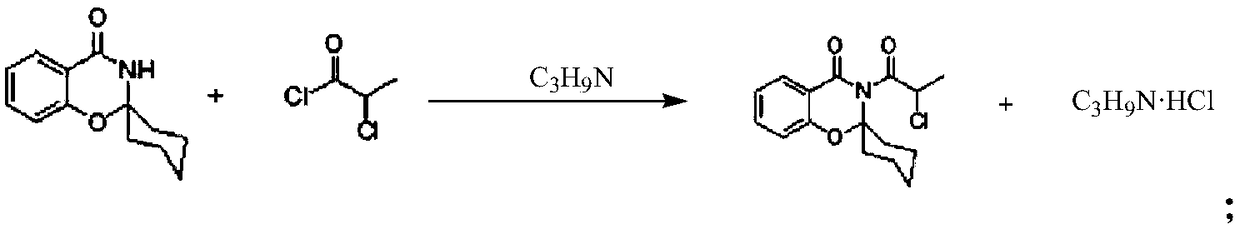
[0028] 2. Synthesis of the second step:
[0029] Put toluene into the reaction kettle, raise the temperature to 100°C until the material dissolves, then keep stirring for 10 minutes, cool dow...
Embodiment 2
[0032] 1. Synthesis of the first step:
[0033] In the reactor, put toluene first, then drop into 137.14g salicylamide, 6.89g p-toluenesulfonic acid, and 166.84g cyclohexanone, and heat to reflux for 3 hours. After the reflux, cool down to 10-15°C and keep warm for 1 hour, centrifuge to shake the material, wait for drying, then rinse with toluene, and then dry. The finished product (weight after drying) of white crystals was obtained. The HPLC content is above 99%. M1 mother liquor is pumped to atmospheric pressure distillation in the kettle until the residual liquid is basically free of toluene, and then the cyclohexanone content in the distillate is detected by the external standard method, which is applied to the next batch, and the residue in the kettle is disposed of in barrels.
[0034] 2. Synthesis of the second step:
[0035] Put toluene into the reaction kettle, raise the temperature to 100°C until the material dissolves, then keep stirring for 10 minutes, cool dow...
Embodiment 3
[0038] 1. Synthesis of the first step:
[0039] In the reactor, first put toluene, then drop into 137.13g salicylamide, 5.17g p-toluenesulfonic acid, 147.21g cyclohexanone, and heat to reflux for 3 hours. After the reflux, cool down to 10-15°C and keep warm for 1 hour, centrifuge to shake the material, wait for drying, then rinse with toluene, and then dry. The finished product (weight after drying) of white crystals was obtained. The HPLC content is above 99%. M1 mother liquor is pumped to atmospheric pressure distillation in the kettle until the residual liquid is basically free of toluene, and then the cyclohexanone content in the distillate is detected by the external standard method, which is applied to the next batch, and the residue in the kettle is disposed of in barrels.
[0040] 2. Synthesis of the second step:
[0041] Put toluene into the reaction kettle, raise the temperature to 100°C until the material dissolves, then keep stirring for 10 minutes, cool down to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com