Meropenem sodium/tazobactam sodium medicinal composition
A technology of tazobactam sodium and meropenem, applied in the field of medicine, can solve the problems of multidrug-resistant Acinetobacter baumannii invasion and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
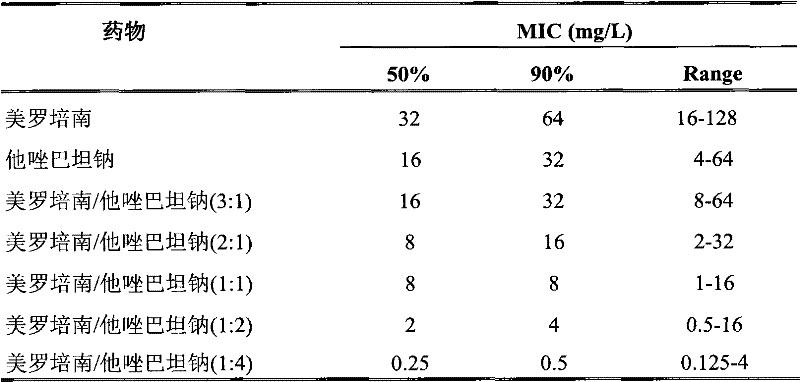
[0030] Example 1: Study on antibacterial effect of meropenem / tazobactam sodium in vitro
[0031] 1. Materials and methods
[0032] 1. Test drugs:
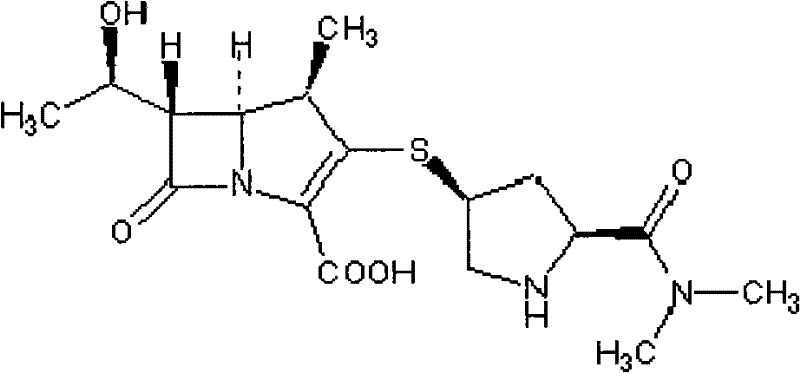
[0033] (1) Meropenem (Meropenem, chemical structure is C17H25N3O5S 3H2O), chemical name 3-[[5-[(dimethylamino)carbonyl]-3-pyrrolidinyl]thio]-6-(1-hydroxyethyl Base)-4-methyl-7-oxo-1-azabicyclo[3,2,0]hept-2-ene-2-carboxylic acid provided by Shenzhen Xintai Pharmaceutical Co., Ltd. Place. Lot No. 130506-200702, Potency: 93.7%
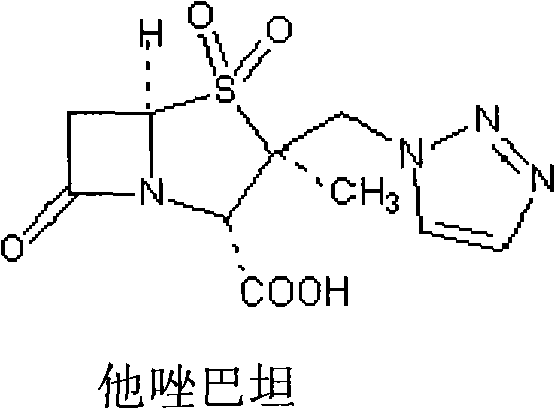
[0034] (2) Tazobactam Sodium (Tazobactam Sodium): batch number 0481-9801, potency: 93.7%. , purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0035] The following proportions are calculated by weight, where the weight of meropenem is calculated based on the dry conversion of meropenem, and the weight of tazobactam is calculated based on the dry conversion of tazobactam sodium.
[0036] 2. Test ratio:
[0037] (1) Meropenem
[0038] (2) Tazobactam sodium
[003...
Embodiment 2
[0075] Example 2: In vivo antibacterial effect of meropenem / tazobactam sodium for injection
[0076] Compositions of meropenem and tazobactam sodium in weight ratio (3:1), (2:1), (1:1), (1:2), (1:4) have certain effects on infected mice Significant antibacterial therapeutic effect.
[0077] 1. Test drug
[0078] Meropenem (Meropenem, molecular formula C17H25N3O5S 3H2O) batch number: 081212; provided by Shenzhen Xintai Pharmaceutical Co., Ltd., purchased from China Institute for the Control of Pharmaceutical and Biological Products; tazobactam sodium: batch number 0481-9801, potency: 93.7%, purchased from From the National Institute for the Control of Pharmaceutical and Biological Products.
[0079] 2 Drug preparation
[0080] Use 0.9% sodium chloride injection solution to prepare different proportions to the required concentration.
[0081] (1), meropenem (single dose)
[0082] (2), tazobactam sodium (single dose)
[0083] (3), meropenem / tazobactam sodium (3:1)
[0084]...
Embodiment 3
[0100] Example 3: Comparative study on the antibacterial effect of meropenem / tazobactam and cefoperazone / tazobactam on Acinetobacter baumannii
[0101] The combination of meropenem and tazobactam sodium in weight ratio (2:1, 1:1, 1:2, 1:4) has significant antibacterial therapeutic effect on infected mice.
[0102] 1. Test drug
[0103]Meropenem (Meropenem, chemical structure C17H25N3O5S 3H2O) was provided by Shenzhen Xintai Pharmaceutical Co., Ltd. and purchased from China National Institute for the Control of Pharmaceutical and Biological Products.
[0104] Cefoperazone / sulbactam sodium (1:1), produced by Pfizer Pharmaceutical Co., Ltd., batch number, 058354030. Provided by Shenzhen Xintai Pharmaceutical Co., Ltd.
[0105] Tazobactam sodium: batch number 0481-9801, potency: 93.7%. purchased from China National Institute for the Control of Pharmaceutical and Biological Products.
[0106] Small white mice, Kunming species, weighing 18-22g, half male and half male. Provided ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com