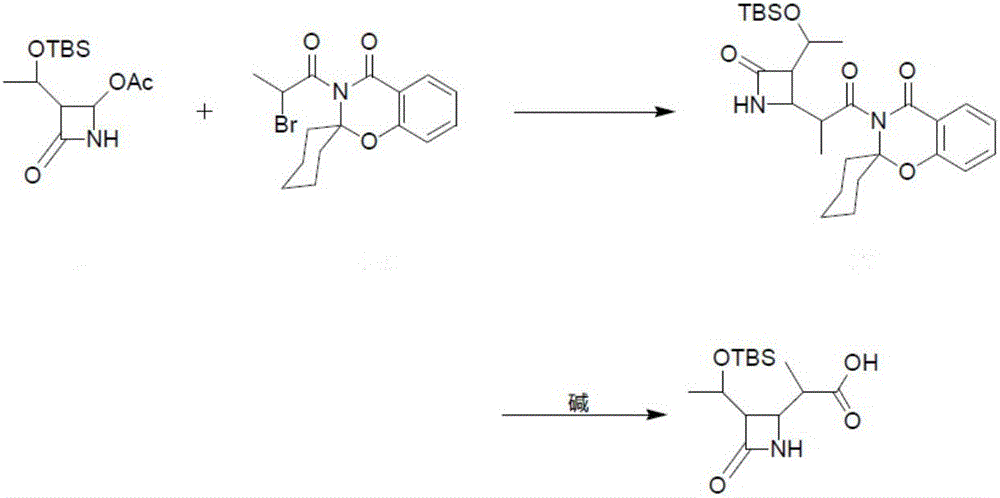
Preparation method of meropenem intermediate 4-BMA
A technology of meropenem and 4-BMA, applied in the field of preparation of meropenem intermediate 4-BMA, can solve the problems of unsatisfactory purity and yield, affecting the quality and cost of meropenem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
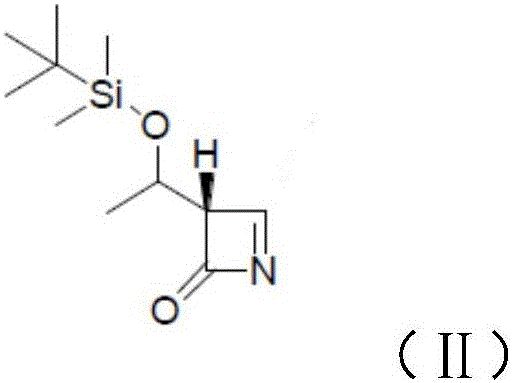
[0023] The present invention provides a preparation method of meropenem intermediate 4-BMA, comprising the following steps:
[0024] A) mixing 4-acetoxyazetidinone, an aprotic solvent and an activated metal to carry out a first reaction;
[0025] B) mixing the product of the first reaction with chloropropionyl spirobenzoxazine cyclohexane to carry out the second reaction;
[0026] C) hydrolyzing the product of the second reaction under hydrogen peroxide and alkaline conditions to obtain 4-BMA.
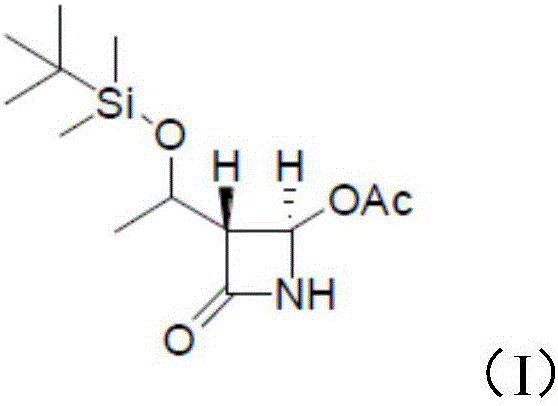
[0027] The 4-acetoxyazetidinone has a structure as shown in formula (I):
[0028]
[0029] The aprotic solvent is preferably one or more of tetrahydrofuran, ethyl acetate, isopropyl ether, benzene, toluene, xylene, chlorobenzene, N,N-dimethylformamide, and dimethyl sulfoxide.
[0030] The activated metal is preferably one or both of magnesium powder and zinc powder.
[0031] In the present invention, the mass ratio of the 4-acetoxyazetidinone to the aprotic solvent is preferably ...
Embodiment 1
[0080] According to the molar ratio of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, α-chloropropionyl chloride and triethylamine is 1:1.4 : 1.2, respectively weigh 80g of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, 65g of α-chloropropionyl chloride and 45g triethylamine; according to the mass ratio of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one to toluene as 1:3.1, weigh 250g toluene; according to the mass ratio of [2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one to isopropanol is 1:1, weigh 80g isopropanol.
[0081] Under nitrogen protection, toluene, spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, α-chloropropionyl chloride and triethylamine Put it into a four-neck flask for reaction, the reaction temperature is 80°C, and the reaction time is 5h. After the reaction, cool down to room temperature, wash three times with 200g water, three times with 200g 8% sodium hydroxide solution, three times with 200g wa...
Embodiment 2
[0083] According to the molar ratio of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, α-chloropropionyl chloride and pyridine is 1:1.6:1.4 , weigh 80g of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, 75g of α-chloropropionyl chloride and 40g of pyridine ;According to the mass ratio of spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one to chlorobenzene is 1:4.4, weigh 350g chlorobenzene ; According to the mass ratio of [2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one to ethanol is 1:1, weigh 80g of ethanol.
[0084] Under nitrogen protection, chlorobenzene, spiro[2,3-dihydro-4H-1,3-benzoxazin-2,1'-cyclohexane]-4-one, α-chloropropionyl chloride and pyridine were added The reaction was carried out in a four-neck flask, the temperature of the reaction was 50° C., and the reaction time was 8 h. After the reaction, cool down to room temperature, wash three times with 200g water, three times with 200g 10% potassium hydroxide solution, thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com