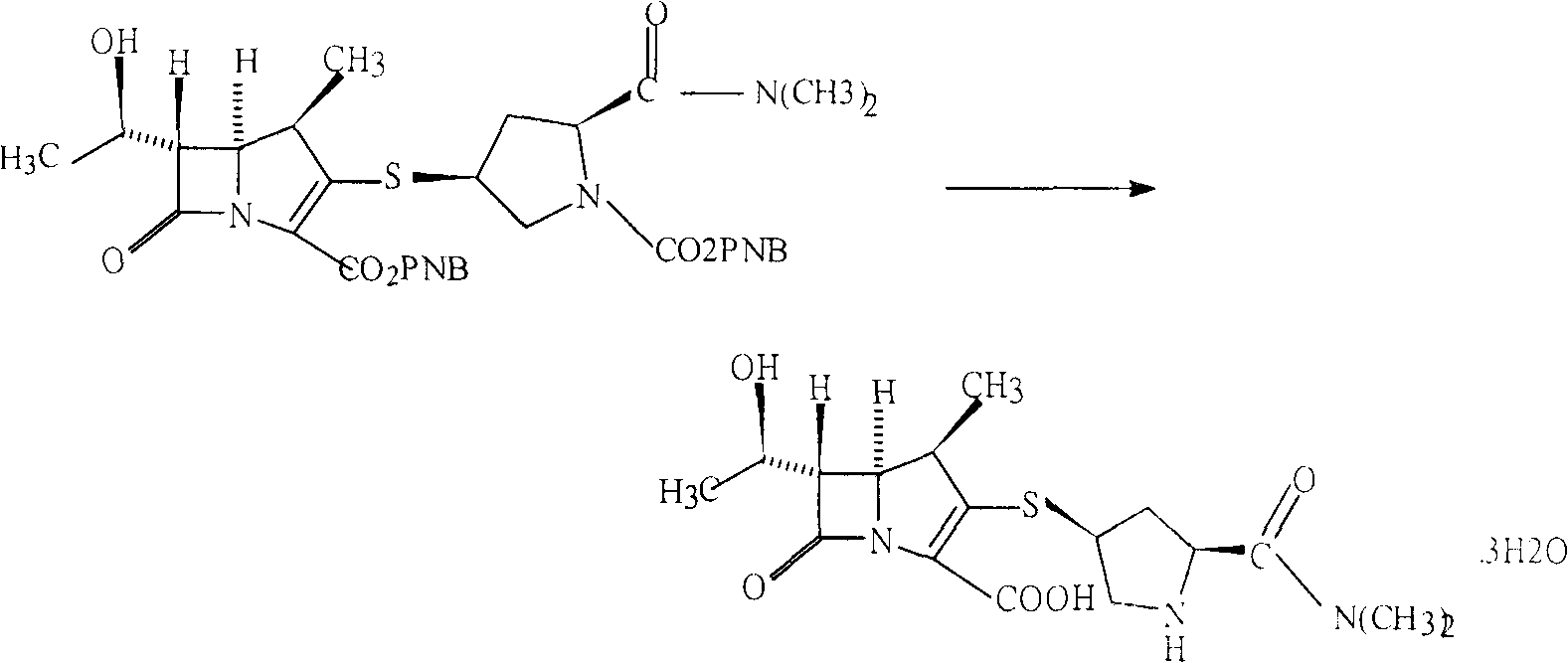
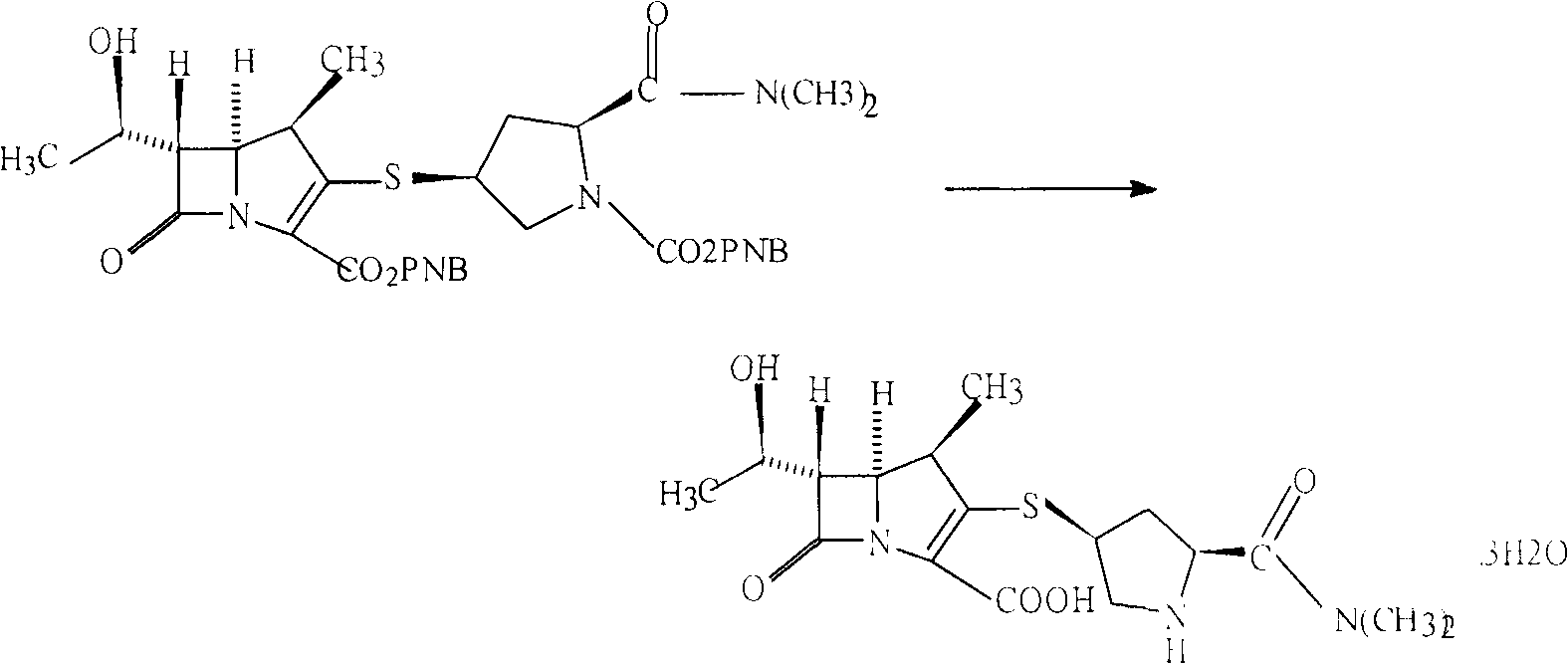
Deprotection method in meropenem synthesis
A technology of meropenem and deprotection group, applied in the field of deprotection group in the synthesis of meropenem, can solve the problems of low weight yield, high equipment and operation requirements, and achieve the effects of high yield, low equipment investment and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 500ml four-neck flask, add 175ml of tetrahydrofuran, slowly add 11g of protected meropenem while stirring, dissolve until clear, add 150g of pure water and 5g of ammonium formate while stirring;
[0020] Slowly raise the temperature to reflux, stir and reflux for 30 minutes, cool to normal temperature, add measured 1.5g of palladium catalyst and 5g of pure water and mix evenly into the flask, wash with a small amount of water and put into the flask. Slowly warming up to reflux again, reflux reaction for 2 hours;
[0021] Cool to normal temperature, vacuum filter, wash the filter cake with hot water at 90°C, and drain it. Filtrate is transferred in the 2000ml three-neck flask;
[0022] Quickly add 500g of acetonitrile into the three-necked flask, and cool down to 10--15°C. Within 10-15 minutes, 600g of acetonitrile was added dropwise under rapid stirring, and the temperature was controlled at 10--15°C. Stir to grow crystals for 1 hour. Suction filtration, the fi...
Embodiment 2
[0024] In a 1000ml four-neck flask, add 350ml of methanol, slowly add 22g of protected meropenem under stirring, dissolve until clear, add 300g of pure water and 8g of formic acid under stirring;
[0025] Slowly heat up to reflux, stir and reflux for 30 minutes, cool to normal temperature, add metered 4g of 10% palladium carbon catalyst and 10g of pure water and mix evenly into the flask, wash with a small amount of water and put into the flask. Slowly warming up to reflux again, reflux reaction for 2 hours;
[0026] Cool to normal temperature, vacuum filter, wash the filter cake with hot water at 90°C, and drain it. Filtrate is transferred in the 5000ml three-neck flask;
[0027] Quickly add 1000g of acetone to the three-necked flask, and cool down to 10--15°C. Within 10-15 minutes, add 1200g of acetone dropwise under rapid stirring, and control the temperature at 10--15°C. Stir to grow crystals for 1 hour. Suction filtration, the filter cake was rinsed with 50g of aceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com