Method for preparing meropenem
A technology of preparation steps and main raw materials, applied in the field of organic synthesis and preparative chemistry, can solve the problems of low yield, unsuitable for large-scale production, low-temperature storage, etc., and achieve the effects of stable process, cost saving of raw materials, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
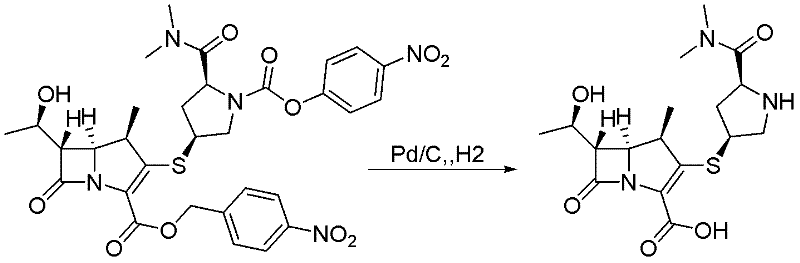
[0036] Embodiment 1: A method for preparing meropenem, characterized in that the specific preparation steps are as follows:
[0037] Add 20 kg (5 vol) of N-methylmorpholine and acetic acid buffer solution with pH = 6.8 into a 100L reactor, cool down to 0±2°C, add 2 kg (0.5 g / g) of 10% platinum carbon, 2-methyl Add 17.8kg of the main raw material (4kg, 1eq) solution diluted with tetrahydrofuran (4vol) dropwise to 7.6kg of oxalic acid (8eq) solution diluted with 2-methyltetrahydrofuran (1vol), and keep warm at 0±2°C for 5h until the reaction is complete. After the reaction is complete, filter with suction, extract the filtrate twice with 15kg (5vol*2) of methyl tert-butyl ether, add 200g (0.05g / g) of activated carbon to the water phase for decolorization, filter, and add 320kg (100vol) of acetone dropwise to the water phase Crystallization, the crude product of meropenem was dissolved and separated by membrane to obtain 1.43 kg of meropenem, with a yield of 65% and a purity of 9...
Embodiment 2
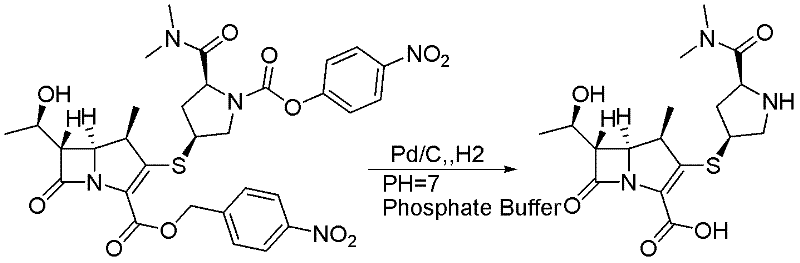
[0038] Embodiment 2: A method for preparing meropenem, characterized in that the specific preparation steps are as follows:
[0039]Add 20 kg (10 vol) of N-methylmorpholine and acetic acid buffer solution of pH = 7.5 into the 100 L reaction kettle, cool to 10 ± 2 ℃, add 1.2 kg (0.6 g / g) of 10% palladium carbon successively, tetrahydrofuran (8 vol ) diluted main raw material (2kg, 1eq) solution 16.2kg, add dropwise 5.1kg of formic acid (12eq) solution diluted with tetrahydrofuran (2vol), and keep warm at 10±2°C for 4.5h until the reaction is complete. After the reaction is completed, filter with suction, extract the filtrate twice with 11kg (6vol*2) of tetrahydrofuran, add 160g (0.08g / g) of activated carbon to the water phase for decolorization, filter, and crystallize by adding 237kg (150vol) of acetonitrile dropwise to the water phase to obtain the 880g of meropenem was obtained by membrane separation after the crude product was dissolved, with a yield of 80% and a purity of ...
Embodiment 3
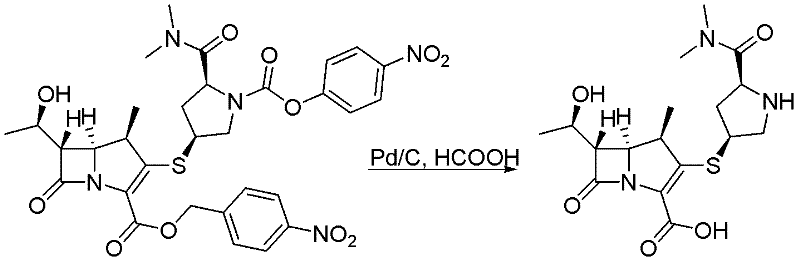
[0040] Embodiment 3: A method for preparing meropenem, characterized in that the specific preparation steps are as follows:
[0041] Add 4.8kg (15vol) of N-methylmorpholine and acetic acid buffer solution at pH = 7.8 to a 20L reactor, and add 320g (1g / g) of Raney nickel and methyl tert-butyl ether in sequence at 15±2°C (10vol) diluted main raw material (320g, 1eq) solution 2.7kg, add dropwise 1.7kg of glyoxylic acid (15eq) solution diluted with methyl tert-butyl ether (5vol), and keep warm at 15±2°C for 4.5h to The response is complete. After the reaction is complete, filter with suction, extract the filtrate twice with 2.8kg (10vol*2) of 2-methyltetrahydrofuran, add 32g (0.1g / g) of activated carbon to the water phase for decolorization, filter, and add 57kg (200vol) of tetrahydrofuran dropwise to the water phase Crystallization, the crude product of meropenem was obtained. After the crude product was dissolved, it was separated by membrane to obtain 132 g of meropenem, with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com