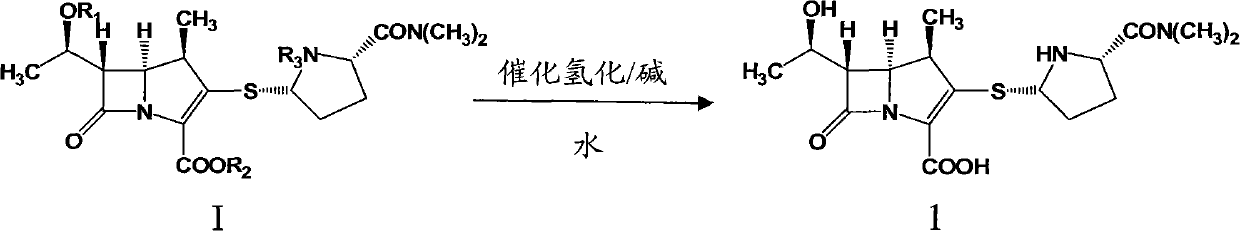
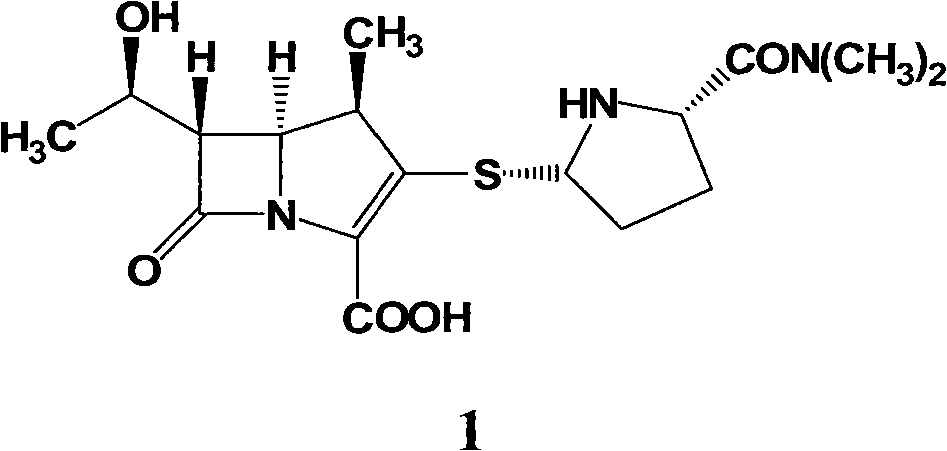
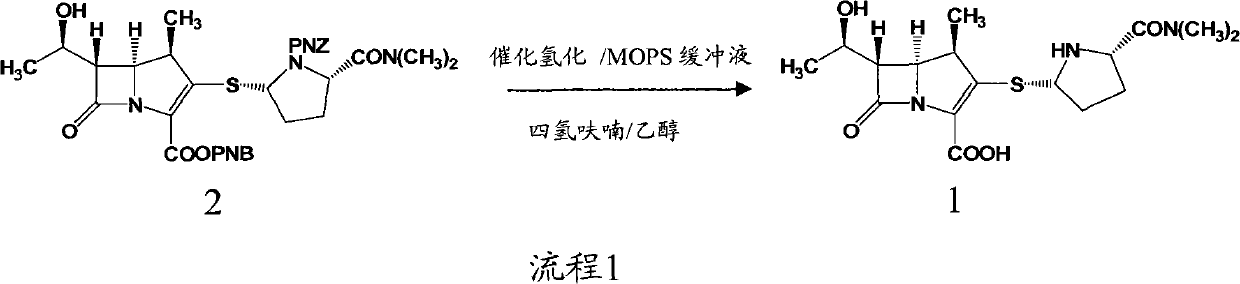
Preparation method of meropenem
A single technology of meropenem, which is applied in the field of preparation of carbapenem compound meropenem, can solve the problems of fast reaction speed, poor product purity, and many degradation products, and achieve the effect of reducing product degradation and improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of Meropenem Trihydrate
[0035] 40.0 L of deionized water, 21.0 Kg (1.43 mol) of meropenem intermediate, 0.46 L (3.97 mol) of 2,6-lutidine, and 0.5 Kg of 10% palladium on carbon were sequentially added into a 100 L hydrogenation reactor. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure of 1.0 MPa in the kettle, the temperature was controlled at 30°C, and stirred for 3 hours. Stirring was stopped, hydrogen was discharged and replaced with nitrogen. Filter and recover the filter cake for reuse; add 160L of acetone to the filtrate, stir and crystallize at 10°C for 4h. After filtration and vacuum drying, 0.425 Kg of off-white solid was obtained, the molar yield was 67.8%, the HPLC purity was 99.4%, and the heavy metal content was less than 10 ppm.
Embodiment 2
[0036] Example 2: Preparation of Meropenem Trihydrate
[0037] 40.0 L of deionized water, 21.0 Kg (1.43 mol) of meropenem intermediate, 0.50 L (4.32 mol) of 2,6-lutidine, and 0.3 Kg of 10% palladium on carbon were sequentially added into a 100 L hydrogenation reactor. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure of 1.5 MPa in the kettle, the temperature was controlled at 20°C, and stirred for 2 hours. Stirring was stopped, hydrogen was discharged and replaced with nitrogen. Filter and recover the filter cake for reuse; add 160L of acetone to the filtrate, stir and crystallize at 10°C for 4h. Filtration and vacuum drying yielded 0.436 Kg of off-white solid with a molar yield of 69.5%, an HPLC purity of 99.2%, and a heavy metal content of <10 ppm.
Embodiment 3
[0038] Example 3: Preparation of Meropenem Trihydrate
[0039] 40.0 L of deionized water, 21.0 Kg (1.43 mol) of meropenem intermediate, 0.65 L (5.61 mol) of 3,5-lutidine, and 0.1 Kg of 10% palladium on carbon were sequentially added into a 100 L hydrogenation reactor. Nitrogen was replaced several times, hydrogen was replaced several times, and finally hydrogen was passed to the pressure of 2MPa in the kettle, the temperature was controlled at 10°C, and stirred for 2h. Stirring was stopped, hydrogen was discharged and replaced with nitrogen. Filter and recover the filter cake for reuse; add 160L of acetone to the filtrate, stir and crystallize at 0°C for 4h. After filtration and vacuum drying, 0.459 Kg of off-white solid was obtained, the molar yield was 73.2%, the HPLC purity was 99.0%, and the heavy metal content was <10 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com