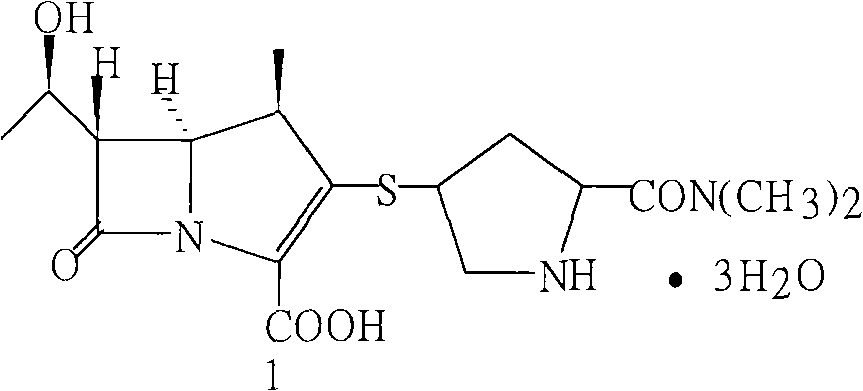
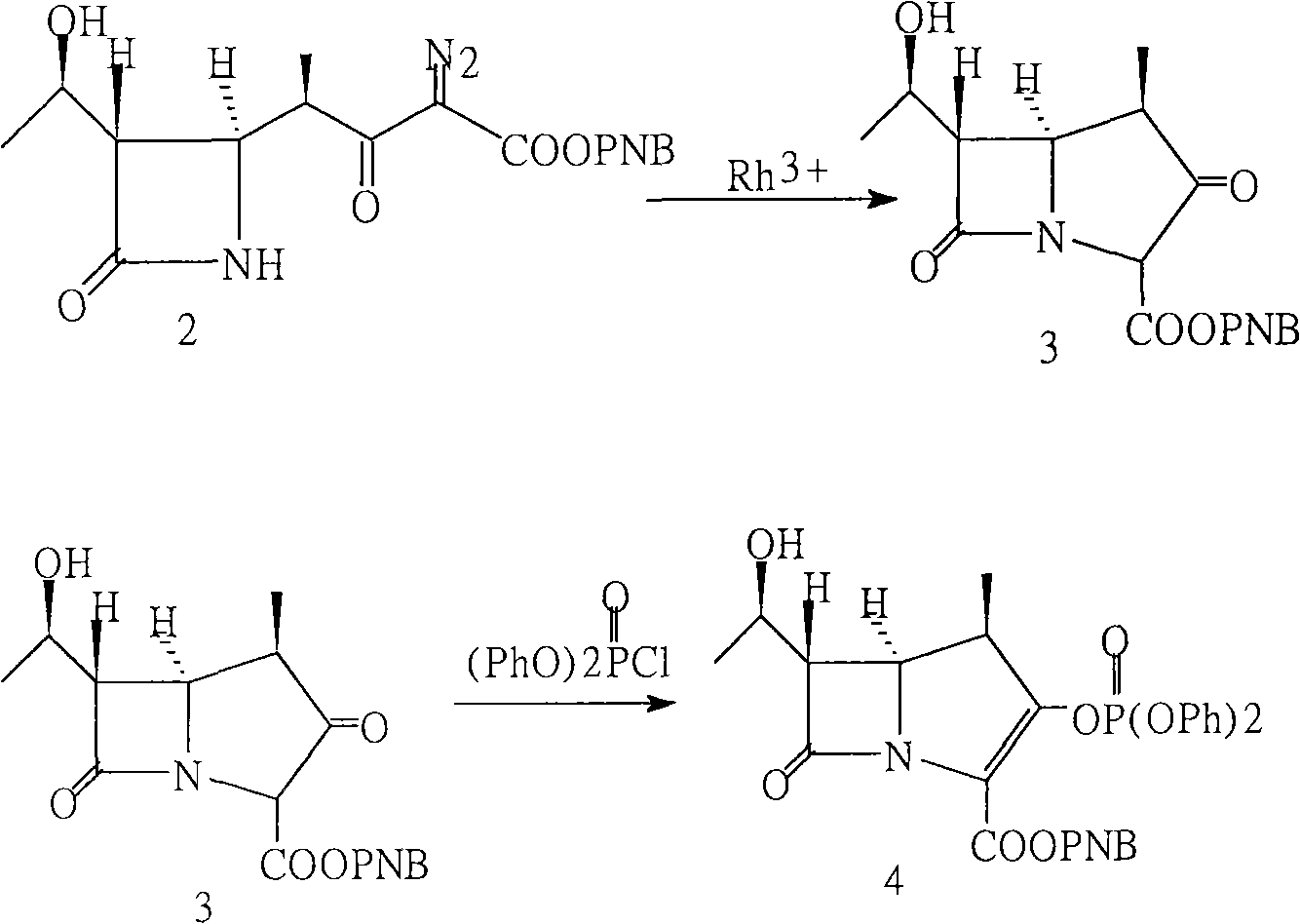
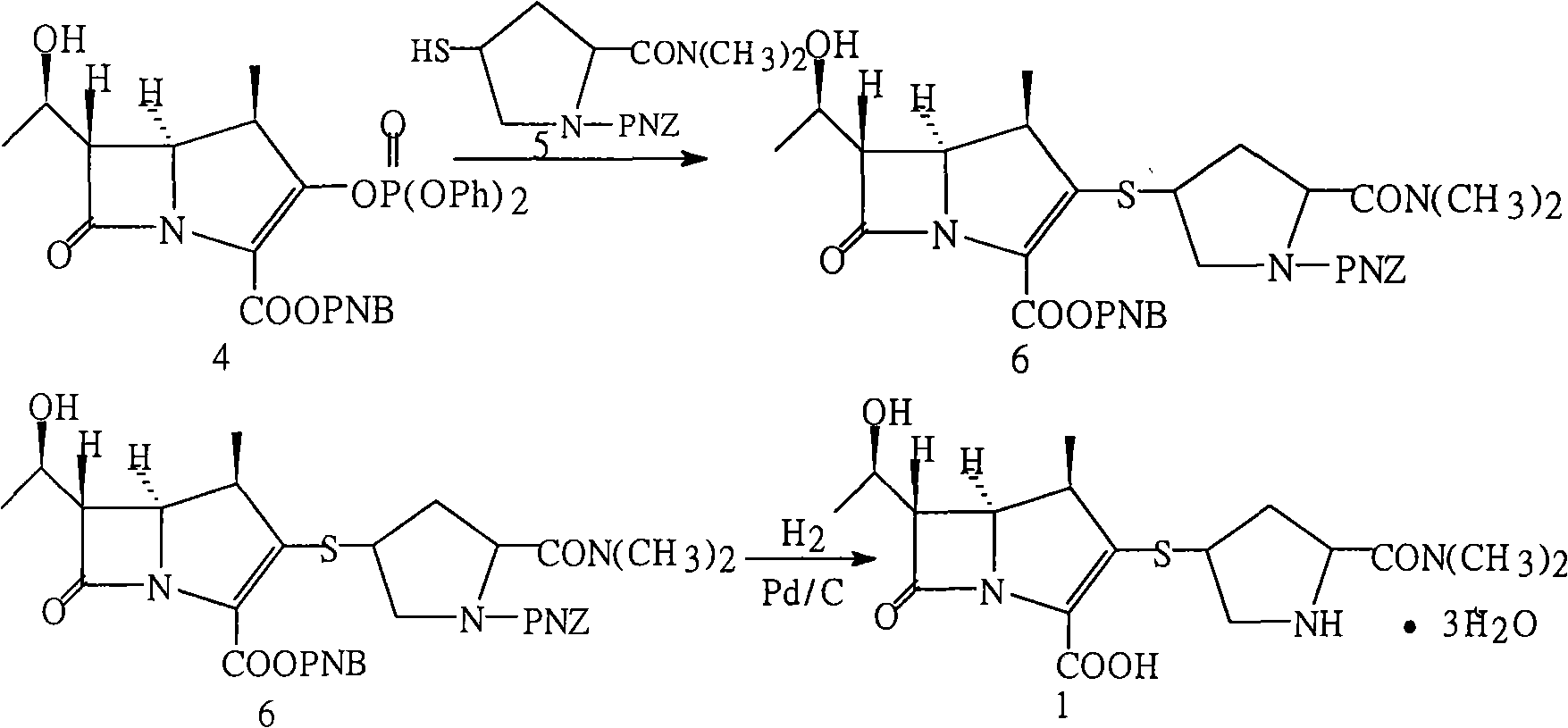
Method for preparing meropenem
A meropenem-specific technology, applied in the field of medicine, can solve problems such as poor production operability and planning, maximize chemical reaction efficiency, and low equipment utilization, so as to improve production operability, ensure product purity, and improve The effect of controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Add 50 g of diazoketone ester to a 1000 ml three-neck bottle connected with an exhaust gas absorption device, add 260 ml of anhydrous ethyl acetate (water content <0.05%), stir to dissolve, heat up to 75 ° C, remove the heat source, and stop Heating, add 0.25g of rhodium octanoate at one time, a large amount of gas overflows, the gas overflow is not obvious after half an hour, take a sample, when the content of diazoketone ester is lower than 0.3%, prepare for the next step reaction, if it is higher than 0.3%, keep it warm The reaction was continued for 30 minutes.
[0016] In another 1000ml three-neck flask, add 300ml of acetonitrile, then pre-cool to -25°C in a low-temperature reactor, add the reaction solution of the cyclization in the previous step at one time, wash and combine with 50ml of acetonitrile, add 20ml of triethylamine, and add diphenyl The oxyphosphoryl ratio is 45ml, adjust the temperature and react at -15°C for 1 hour, take a sample, and whe...
Embodiment 2
[0017] Embodiment 2: (replace rhodium octanoate with rhodium acetate)
[0018] Add 50g of diazoketone ester to a 1000ml three-necked bottle connected with an exhaust gas absorption device, add 260ml of anhydrous ethyl acetate (water content<0.05%), stir to dissolve, heat up to 75°C, remove the heat source, stop heating, disposable Add 0.21g of rhodium acetate, a large amount of gas escapes, and the gas overflow is not obvious after half an hour. Take a sample. When the content of diazoketone ester is lower than 0.3%, prepare for the next step of reaction. If it is higher than 0.3%, keep warm and continue the reaction for 30 minutes .
[0019] In another 1000ml three-neck flask, add 300ml of acetonitrile, then pre-cool to -25°C in a low-temperature reactor, add the reaction solution of the cyclization in the previous step at one time, wash and combine with 50ml of acetonitrile, add 20ml of triethylamine, and add diphenyl The oxyphosphoryl ratio is 45ml, adjust the temperature ...
Embodiment 3
[0020] Example 3: Purification of Meropenem
[0021] Add 10 g of the crude meropenem above into a 500 ml three-necked bottle, add 400 ml of purified water to dissolve, add 1 g of carbon to decolorize for 30 minutes, filter, add the filtrate to a 1000 ml three-necked bottle, add 2000 ml of acetone dropwise at 10-15 ° C, the aqueous solution becomes cloudy, Crystals were precipitated, grown for 30 minutes, filtered, washed with 20ml of acetone, and dried at -0.09kpa / 40°C for 2 hours. The finished product of meropenem was 8.8g, with a yield of 88% and a content of 98.9% (normalized method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com