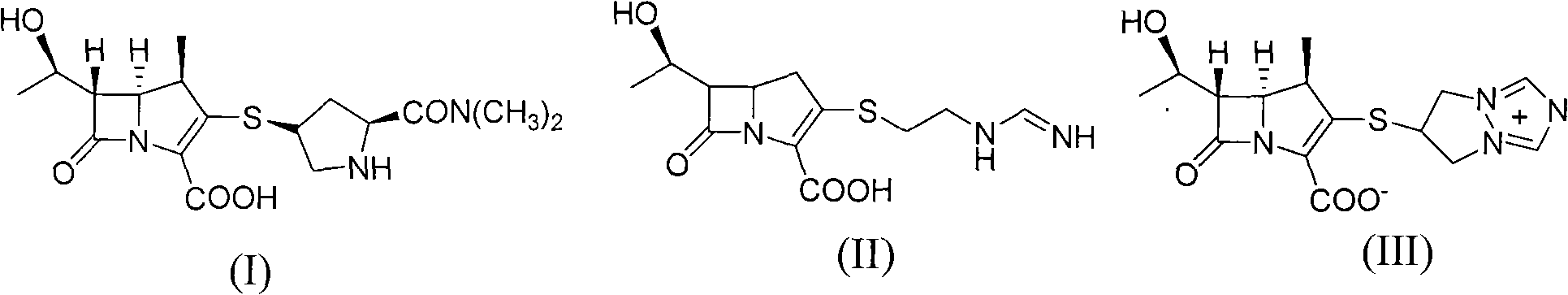
Preparation of meropenem
A technology for meropenem and compounds, which is applied in the directions of organic chemistry, antibacterial drugs, etc., can solve the problems of inconsistent atomic economy, unfavorable sustainable development, and difficulty in obtaining raw materials, and achieves a shortened production cycle, simplified operation, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
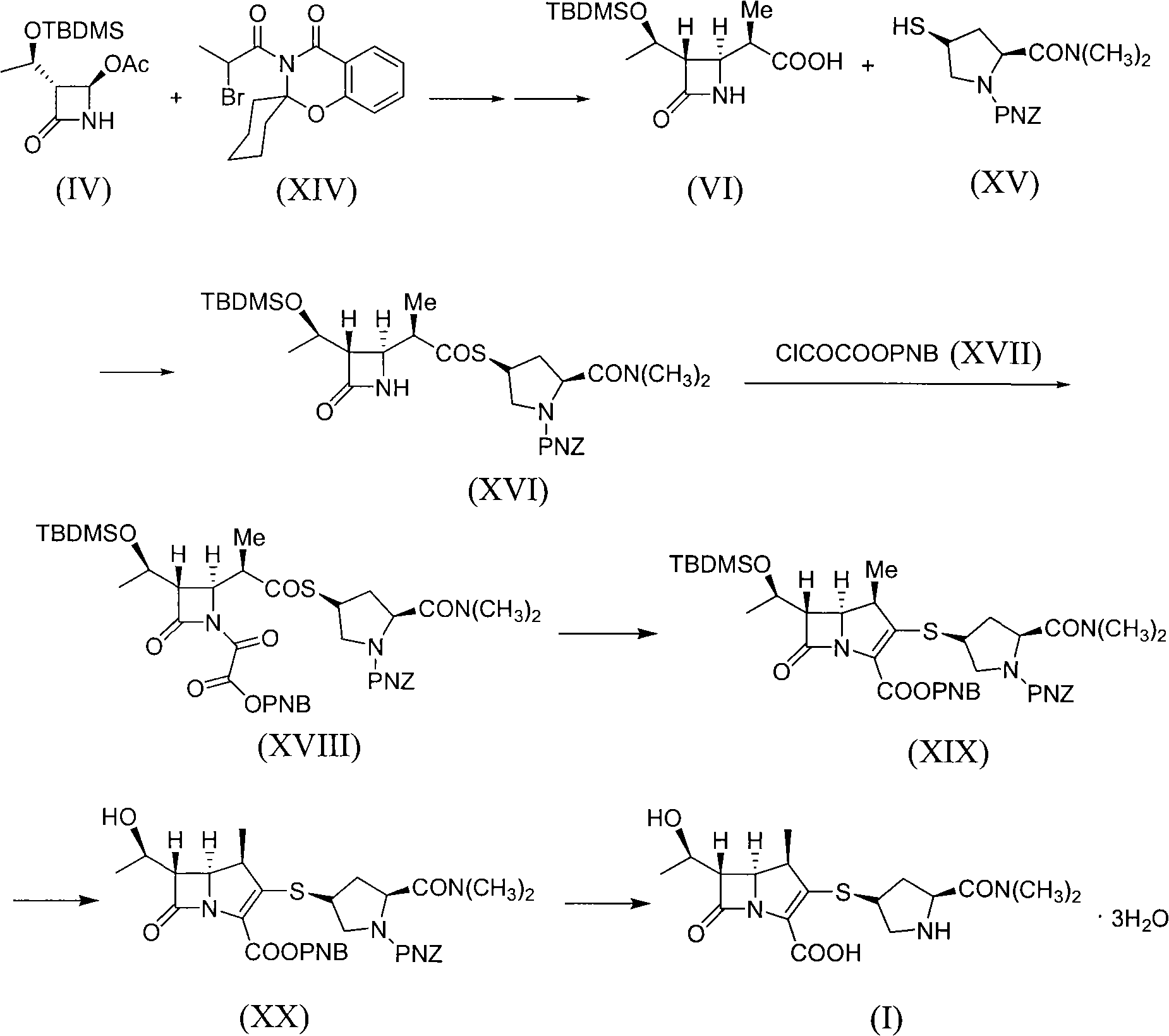
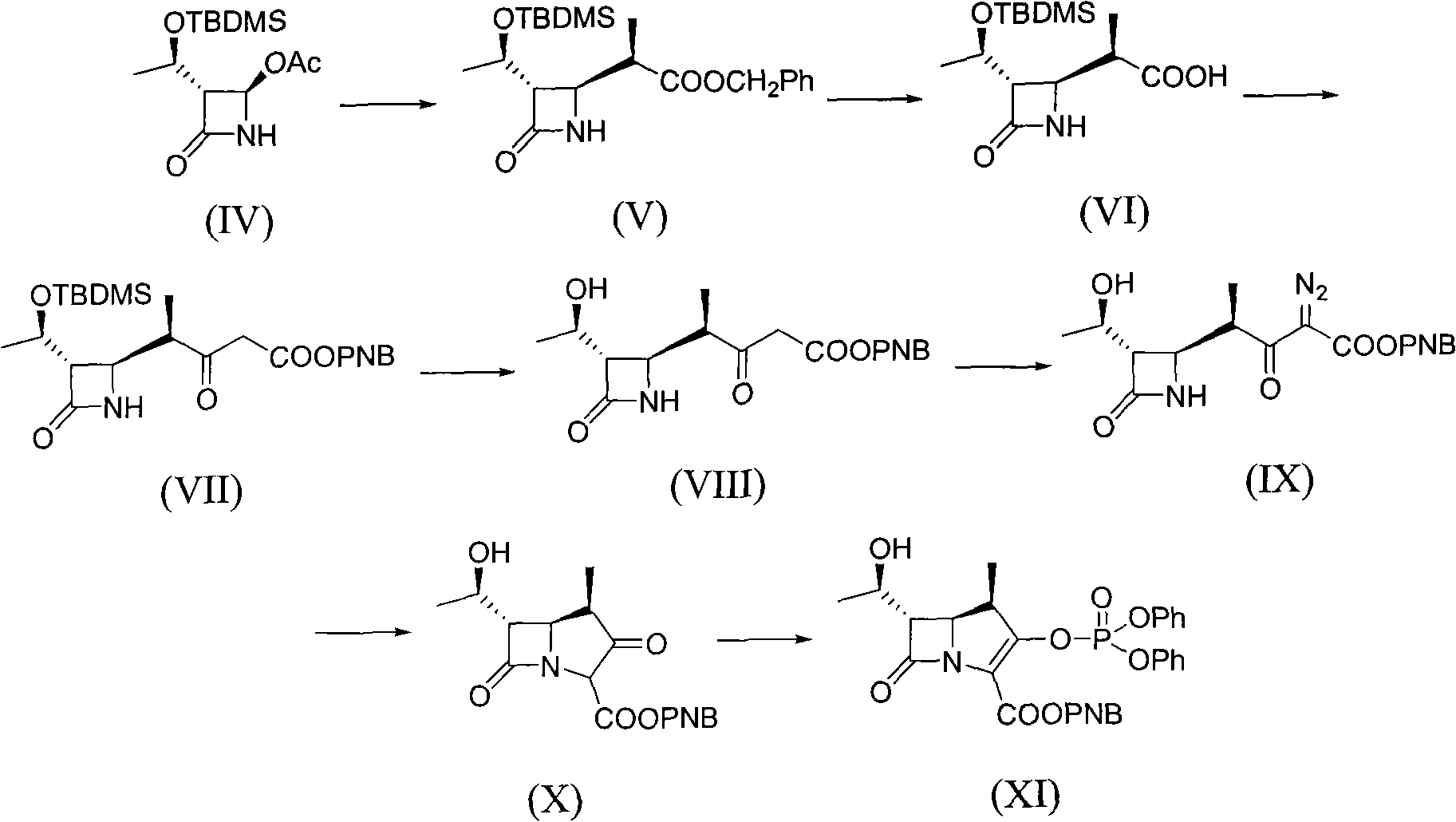
[0036] [Example 1] (3S, 4R)-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4-[(2R)-2-methyl-1-carboxyethyl] -Synthesis of azetidin-2-one (VI)
[0037] Add 120.70g (1.857mol) of zinc powder to 215ml of anhydrous tetrahydrofuran, heat to boiling with stirring, then add 177.50g (0.618mol) (3S, 4R)-4-acetoxy-3-[(1R) -1-tert-Butyldimethylsiloxyethyl]azetidin-2-one (IV) and 319.50 g (0.909 mol) 3-(2-bromopropyl)-spiro[2,3-dihydro -4H-1,3-benzoxazine-2,1'-cyclohexyl]-4-one (XIV) was dissolved in 640ml of anhydrous tetrahydrofuran to form a mixed solution, the rate of addition was such that the reaction solution did not boil, After adding, reflux for 30 minutes, cool down to room temperature, add 18 g of diatomaceous earth to the reaction mixture, filter the reaction mixture with suction, wash the filter residue with an appropriate amount of tetrahydrofuran, combine the filtrate and the washing liquid, add 215 ml of toluene, and add 710 ml of toluene to the mixture. 2N hydrochloric acid, ...
Embodiment 2
[0042] [Example 2] (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsiloxy)ethyl]-4-{(2R)-2-methyl-1-carbonyl Synthesis of Ethyl-[(2S,4R)-4-(1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl)pyrrolidinylthio]}-azetidin-2-one (XVI)
[0043] To 90.3 g (0.30 mol) (3S,4R)-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4-[(2R)-2-methyl-1- Carboxyethyl]-azetidin-2-one (VI) and 68.1 g (0.33 mol) of dicyclohexanediamine (DCC) dissolved in 400 ml of dichloromethane were added 3 g of 4-dimethylaminopyridine (DMAP). ), then 111.30g (0.315mol) (2S, 4S)-2-dimethylaminocarbonyl-4-mercapto-1-(p-nitrobenzyloxycarbonyl)pyrrolidine (XV) was added dropwise to the reaction solution and dissolved in 500ml The solution in dichloromethane was reacted at room temperature for 12 hours. After the completion of the reaction, filter, collect the filtrate, wash the toluene solution with 600ml of 5% acetic acid solution, saturated sodium bicarbonate solution and saturated brine each once, collect the methylene chloride ...
Embodiment 3
[0044] [Example 3] (3S, 4R)-3-[(1R)-1-(tert-butyldimethylsiloxy)ethyl]-4-{(2R)-2-methyl-1-carbonyl Synthesis of Ethyl-[(2S,4R)-4-(1-p-nitrobenzyloxycarbonyl-2-dimethylaminocarbonyl)pyrrolidinylthio]}-azetidin-2-one (XVI)
[0045] 100 g (0.332 mol) (0.30 mol) (3S,4R)-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4-[(2R)-2-methyl at room temperature -1-Carboxyethyl]-azetidin-2-one (VI) and 55.12g (0.349mol) N,N'-carbonyldiimidazole (CDI) were dissolved in 500ml acetone, and then added dropwise to the reaction solution A solution of 111.69g (0.316mol) (2S, 4S)-2-dimethylaminocarbonyl-4-mercapto-1-(p-nitrobenzyloxycarbonyl)pyrrolidine (XV) dissolved in 600ml acetone, react at room temperature for 2.5 hours . After the reaction is completed, acetone is recovered under reduced pressure for recycling, the residual liquid is dissolved in 500 ml of toluene, the toluene solution is washed once with 700 ml of clear water, the toluene layer is collected, dried by adding anhydrous sodium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com