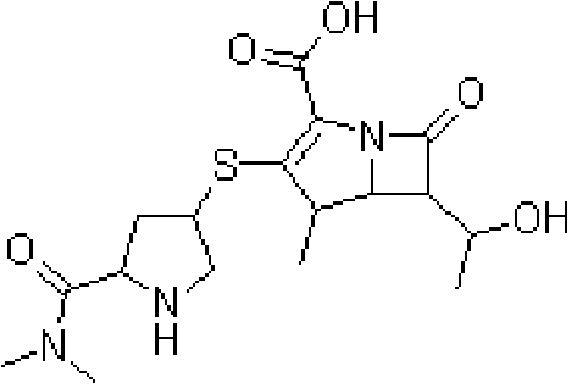
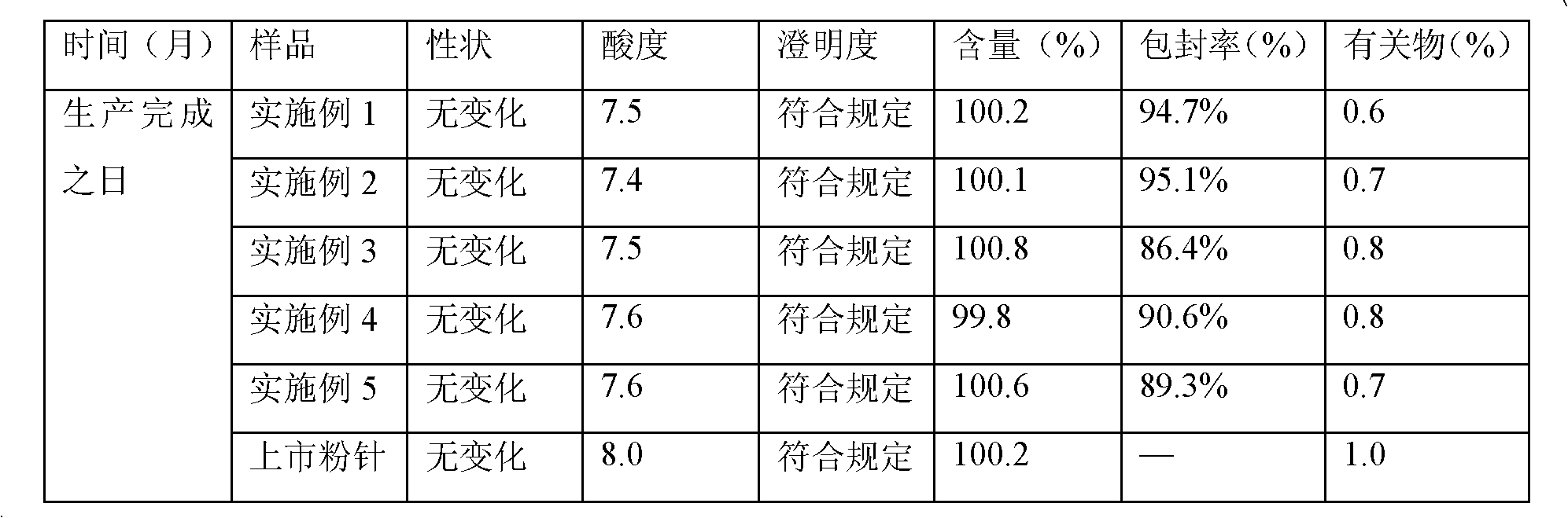
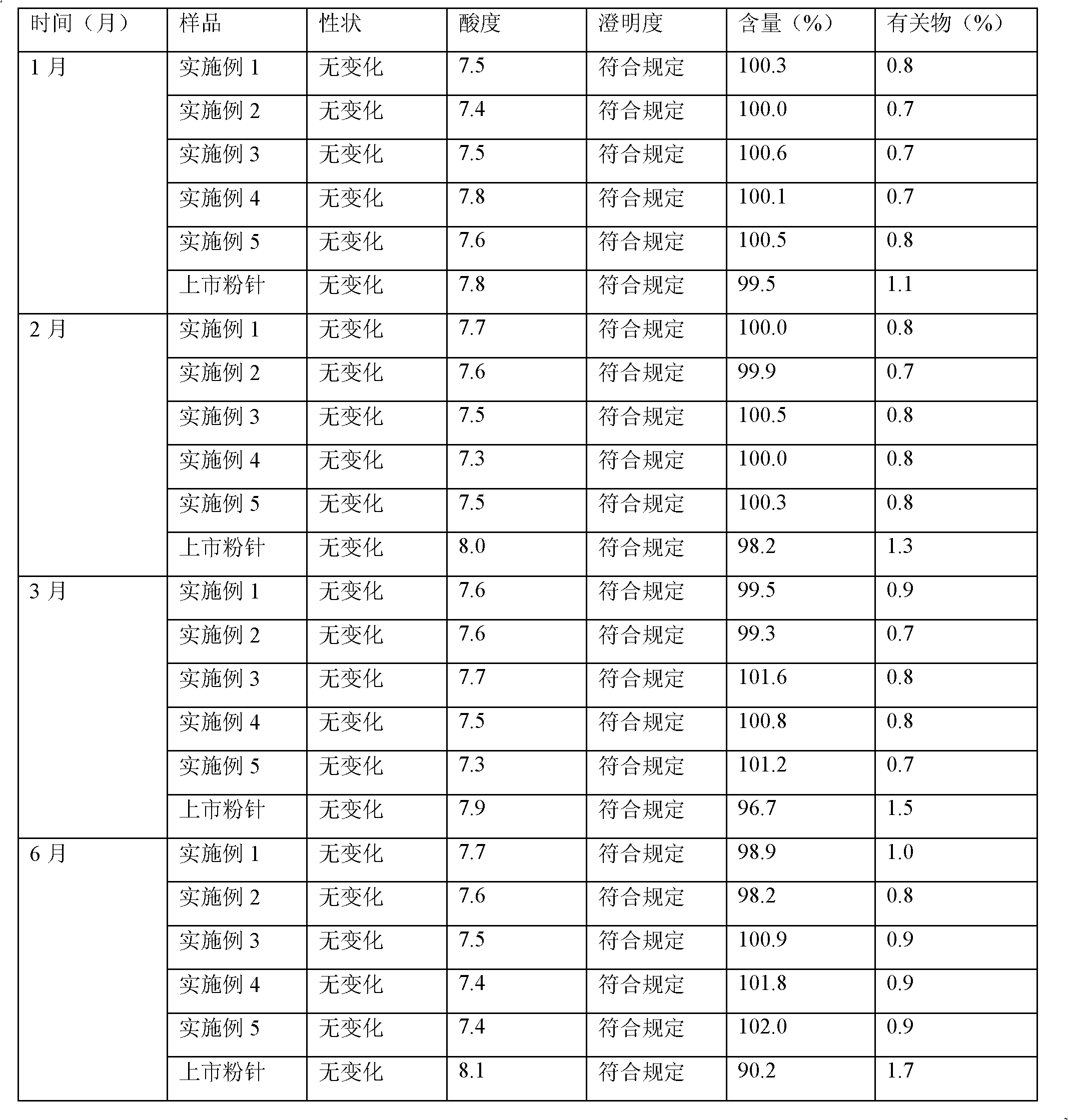
Meropenem freeze-dried preparation for injection and preparation method thereof
A technology for meropenem and meropenem lipid, which is applied in the field of meropenem preparations for injection and their preparation, can solve the problems of poor stability, reduced titer, increased related substances, etc., and achieves stable quality, improved stability, and simple process flow. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation formula: meropenem 12g, soybean oil 80g, refined egg yolk lecithin 60g, glycerin 20g, cyclodextrin 1g, glucose 100g, water for injection 1000ml.
[0032] Specific preparation process: In a preparation tank, disperse 60g of refined egg yolk lecithin and 12g of meropenem in 80g of soybean oil, keep warm at 50°C, stir fully at 2500 rpm for 10min, and mix well to form an oil phase; 20g of glycerin is dispersed in the injection Form the water phase with water; mix the water phase and the oil phase, keep warm at 50-60°C, and stir at 150 rpm for 10 minutes to make a rough milk; and adjust the pH value with 0.2mol / L disodium hydrogen phosphate sodium dihydrogen phosphate buffer 7.5; Homogenize repeatedly through a high-pressure homogenizer to obtain a uniform emulsion. Then, 1 g of β-cyclodextrin was added thereto and dissolved to obtain solution I. After dissolving 100 g of glucose with an appropriate amount of water, solution II was obtained. Stir and mix soluti...
Embodiment 2
[0034] Preparation formula: meropenem 6.7g, soybean oil 30g, refined egg yolk lecithin 25g, glycerin 15g, sodium oleate 2g, cyclodextrin 335mg, glucose 125g, water for injection 1000ml.
[0035] Specific preparation process: In a preparation tank, disperse 25g of refined egg yolk lecithin and 6.7g of meropenem in 30g of soybean oil, keep warm at 60°C, stir fully at 3000 rpm for 10min, and mix to form an oil phase; 15g of glycerin and 2g of Disperse sodium oleate in water for injection to form a water phase; mix the water phase and oil phase, keep warm at 60°C, and stir at 200 rpm for 15 minutes to make a coarse milk; adjust the pH value to 6.5 with citric acid solution; pass through a high-pressure homogenizer Carry out repeated homogenization to obtain a uniform emulsion. When performing homogenization, two-stage homogenization is used, and the pressure of the first-stage valve is 600kg / cm 2 , Secondary valve pressure 120kg / cm 2 , Homogenized 7 times. Then, 335 mg of β-cyc...
Embodiment 3
[0037] Preparation formula: meropenem 25g, olive oil 175g, phosphatidylethanolamine 137.5g, glycerin 50g, sodium oleate 8g, α-cyclodextrin 3g, mannitol 300g, water for injection 2500ml.
[0038]Specific preparation process: In a preparation tank, disperse 137.5g of refined phosphatidylethanolamine and 25g of meropenem in 175g of olive oil, keep warm at 50-60°C, stir fully at 3000 rpm for 10min, and mix well to form an oil phase; 50g of glycerin Disperse with 8g of sodium oleate in water for injection to form a water phase; mix the water phase and oil phase, keep warm at 50-60°C, stir at 200 rpm for 15 minutes, and make a coarse milk; and use 0.2mol / L disodium hydrogen phosphate dibasic acid The pH value of sodium hydrogen buffer is adjusted to 7.5; repeated homogenization is carried out by a high-pressure homogenizer to obtain a uniform emulsion. Then, 3 g of α-cyclodextrin was added thereto for dissolution to obtain solution I. After dissolving 300g of mannitol with an appro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com