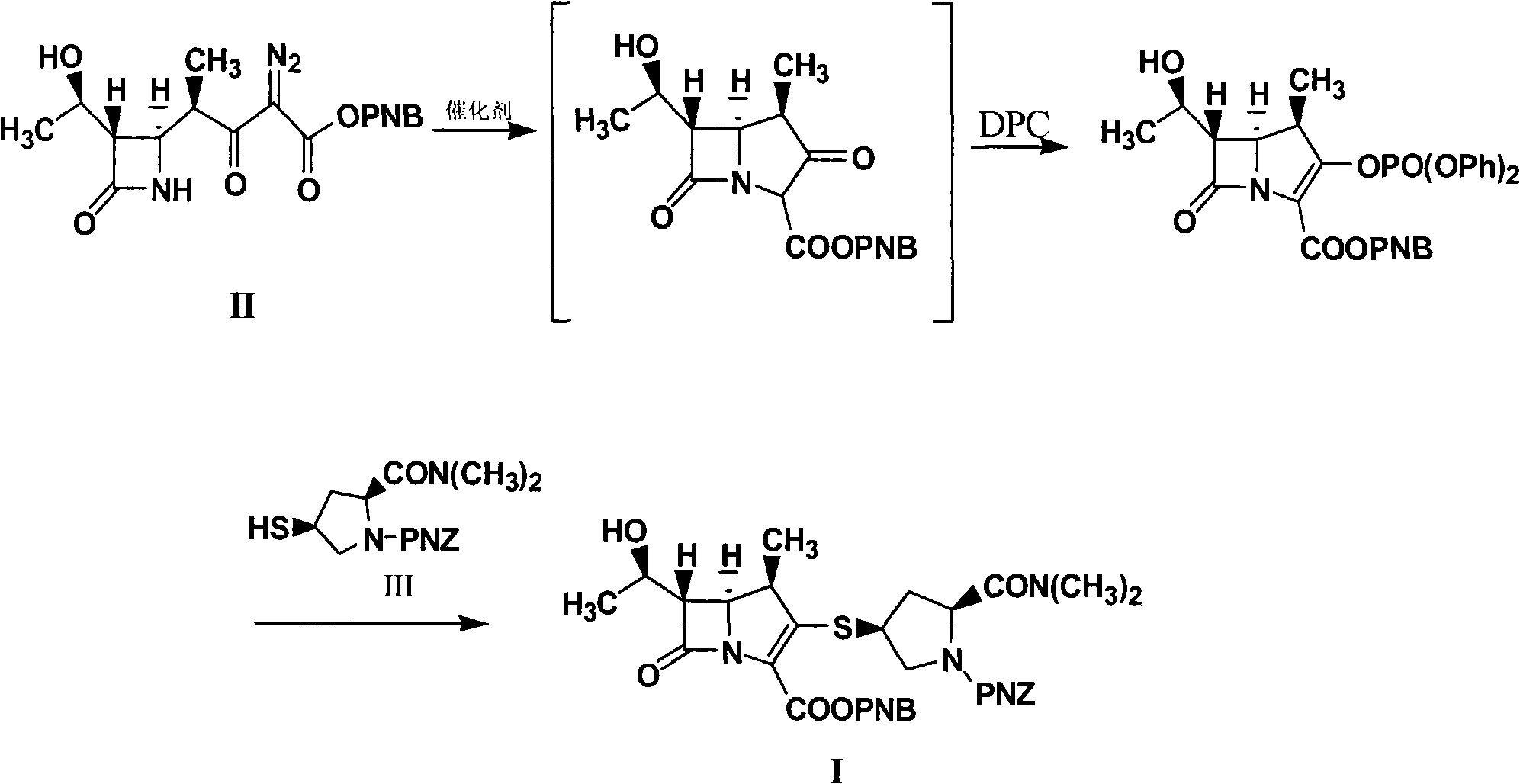
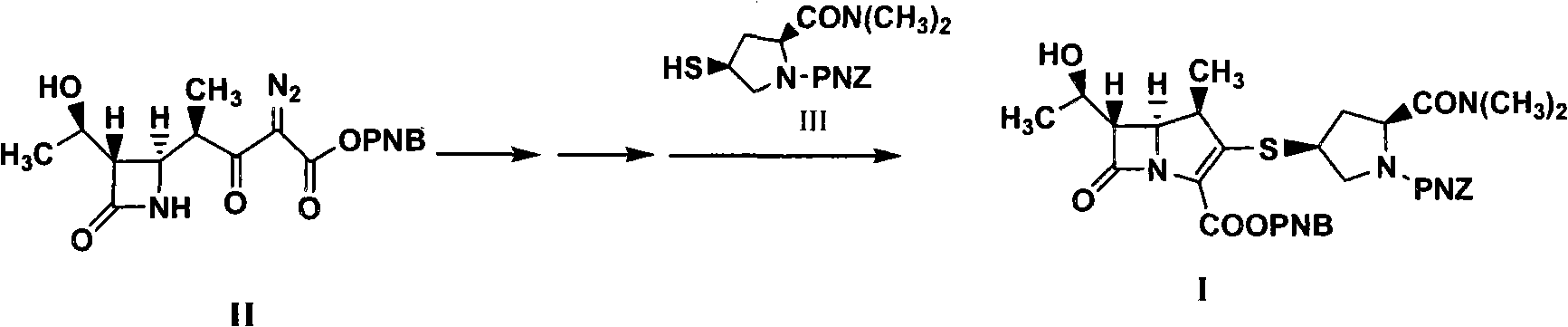
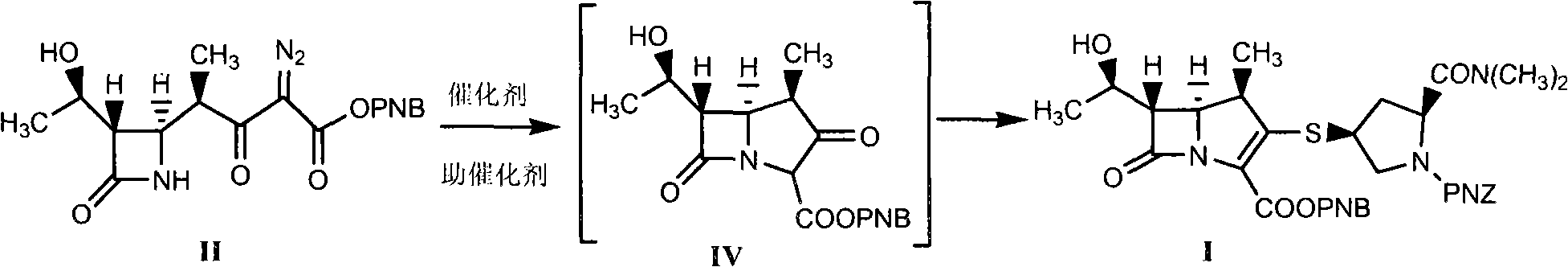
Method for synthesizing meropenem intermediate
A synthesis method, the technology of meropenem, applied in the field of synthesis of meropenem intermediates, can solve the problems of labor-intensive step-by-step operation, uncontrollable yield, increased production cost, etc., to achieve complete reaction of raw materials, high product yield, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Into a reactor equipped with a reflux device, nitrogen gas was preliminarily exhausted to exhaust the air in the reactor, and 20.0 g of compound II (prepared according to patent ZL200610083362.7), 200 ml of dichloromethane, 10 mg of rhodium octanoate, 60 mg of Zinc chloride, heat up to reflux reaction, HPLC monitors the reaction, the reaction is completed, the normalized content of the intermediate product is 93.62%, the obtained reaction solution is cooled to about -20°C, and 150ml of acetonitrile and 10.0g of diisopropyl Diethylethylamine, 30.0g of DPC, react at about -25°C, monitor the reaction by HPLC, after the reaction is completed, cool the obtained reaction solution to about -35°C, add 7.0g of diisopropylethylamine, add 20g of the compound III. Control the temperature and react at about -35°C, monitor by HPLC. After the reaction is completed, add 10% formic acid to wash 8 times, discard the water phase, add 20g of anhydrous magnesium sulfate to the organic phase,...
Embodiment 2
[0049]In the reactor equipped with reflux device, feed nitrogen in advance to exhaust the air in the reactor, add 20.0g of compound II, 200ml of dichloromethane, 10mg of rhodium octanoate, 60mg of zinc bromide, and heat up to reflux reaction, HPLC monitors the reaction. After the reaction is completed, cool the obtained reaction solution to about -20°C, add 150ml of acetonitrile, add 10.0g of diisopropylethylamine, and 30.0g of DPC, react at about -25°C, and monitor by HPLC Reaction, after the reaction is completed, cool down the obtained reaction solution to about -35°C, add 7.0g of diisopropylethylamine, add 20g of compound III, control the temperature to react at about -35°C, monitor by HPLC, after the reaction is complete, add Wash 8 times with 10% sodium dihydrogen phosphate, discard the water phase, add 20 g of anhydrous magnesium sulfate to the organic phase to dry, filter, concentrate the filtrate under reduced pressure, add 200 ml of ethyl acetate to the concentrate to...
Embodiment 3
[0052] In the reactor equipped with a reflux device, feed nitrogen to exhaust the air in the reactor in advance, add 20.0g of compound II, 180ml of methylene chloride, 20mg of rhodium octanoate, 70mg of zinc bromide, and heat up to reflux reaction, HPLC monitors the reaction. After the reaction is completed, cool the obtained reaction solution to about -20°C, add 150ml of acetonitrile, add 7.0g of diisopropylethylamine, 15.0g of DPC, and 30mg of DMAP. The reaction was monitored by HPLC. After the reaction is completed, cool down the obtained reaction solution to about -35°C, add 7.0g of diisopropylethylamine, add 20g of compound III, control the temperature at about -35°C, monitor by HPLC, and add 10% Wash with sodium dihydrogen phosphate 8 times, discard the water phase, add 20 g of anhydrous magnesium sulfate to the organic phase, dry, filter, concentrate the filtrate under reduced pressure, add 200 ml of ethyl acetate to the concentrate to crystallize, filter, and dry the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com