Meropenem raw medicine, preparation method thereof and pharmaceutical composition containing same
A technology of meropenem and API, which is applied in pharmaceutical formulations, antibacterial drugs, medical preparations containing active ingredients, etc. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Add 12 kg of crude meropenem to 120 kg of water, stir to form a suspension, raise the temperature of the material to 50°C to dissolve it, and then lower the temperature to 10°C.
[0085] Add 100 g of activated carbon for decolorization, and filter.
[0086] Add 480 L of crystallization solvent acetonitrile to the filtrate, and stir and grow the crystal for 2 hours after the addition is complete.
[0087] Filter, wash the crystals with acetonitrile, and dry to obtain 10.6 kg of the first refined meropenem.
[0088] The quality of the first refined meropenem: the total amount of impurities is 0.75%; the residual organic solvent is 0.23%.
Embodiment 2
[0090] Add 10 kg of the first refined meropenem product prepared in Example 1 into 150 kg of water, stir, use a heat exchanger to raise the temperature of the feed solution to 50°C, then cool it down to 0°C, and grow the crystal with stirring for 15 hours.
[0091] Filter, wash the crystals with acetonitrile, and dry to obtain 8.1 kg of high-purity meropenem bulk drug.
[0092] Among them, the content of meropenem is 99.6% (calculated as anhydrous matter); impurity A in related substances is 0.04%; impurity B is 0.03%; any unknown single impurity is 0.01%; the sum of other impurities except A and B is 0.02% ; Acetone residue is 100ppm, and acetonitrile is not detected.
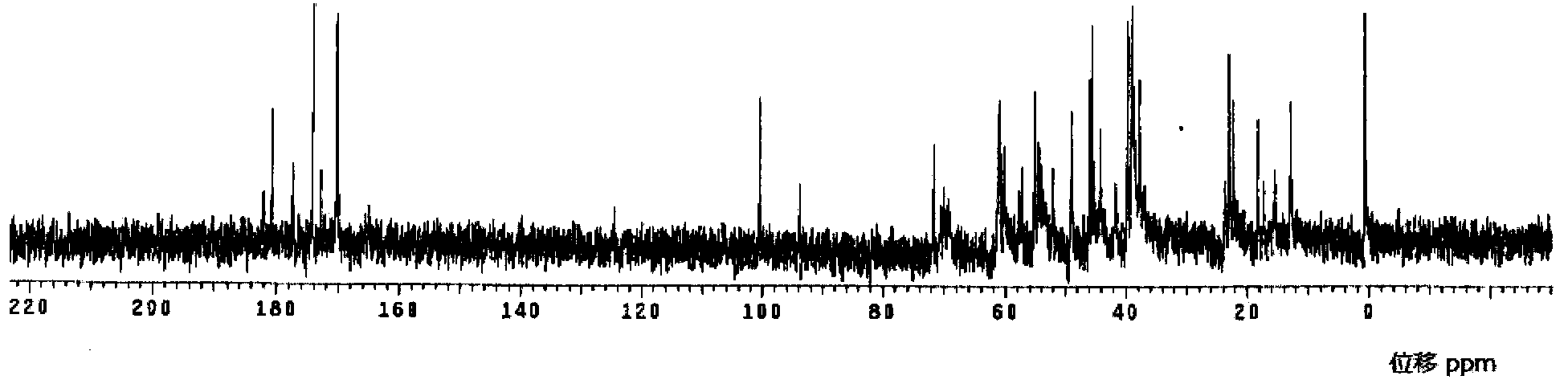
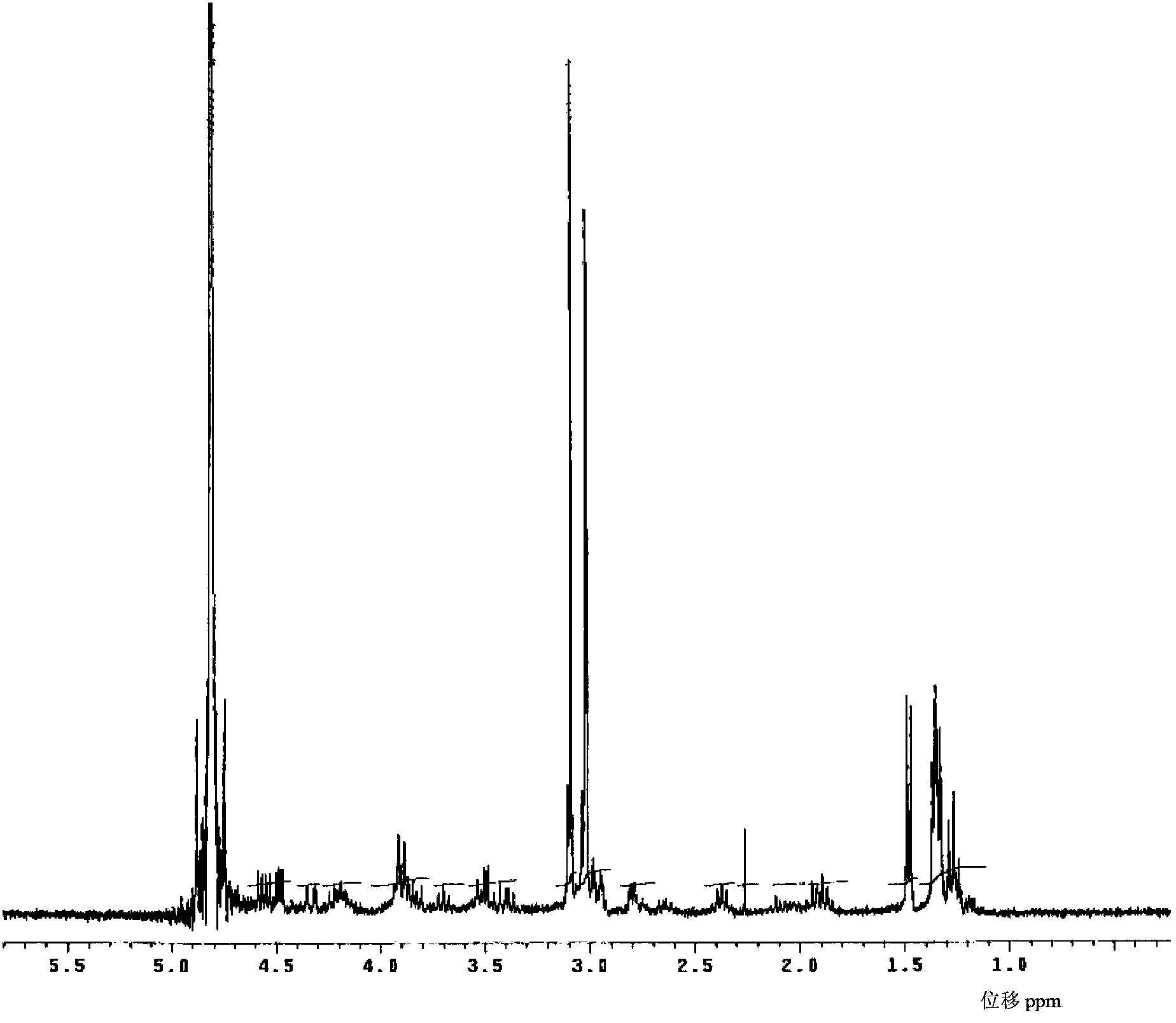
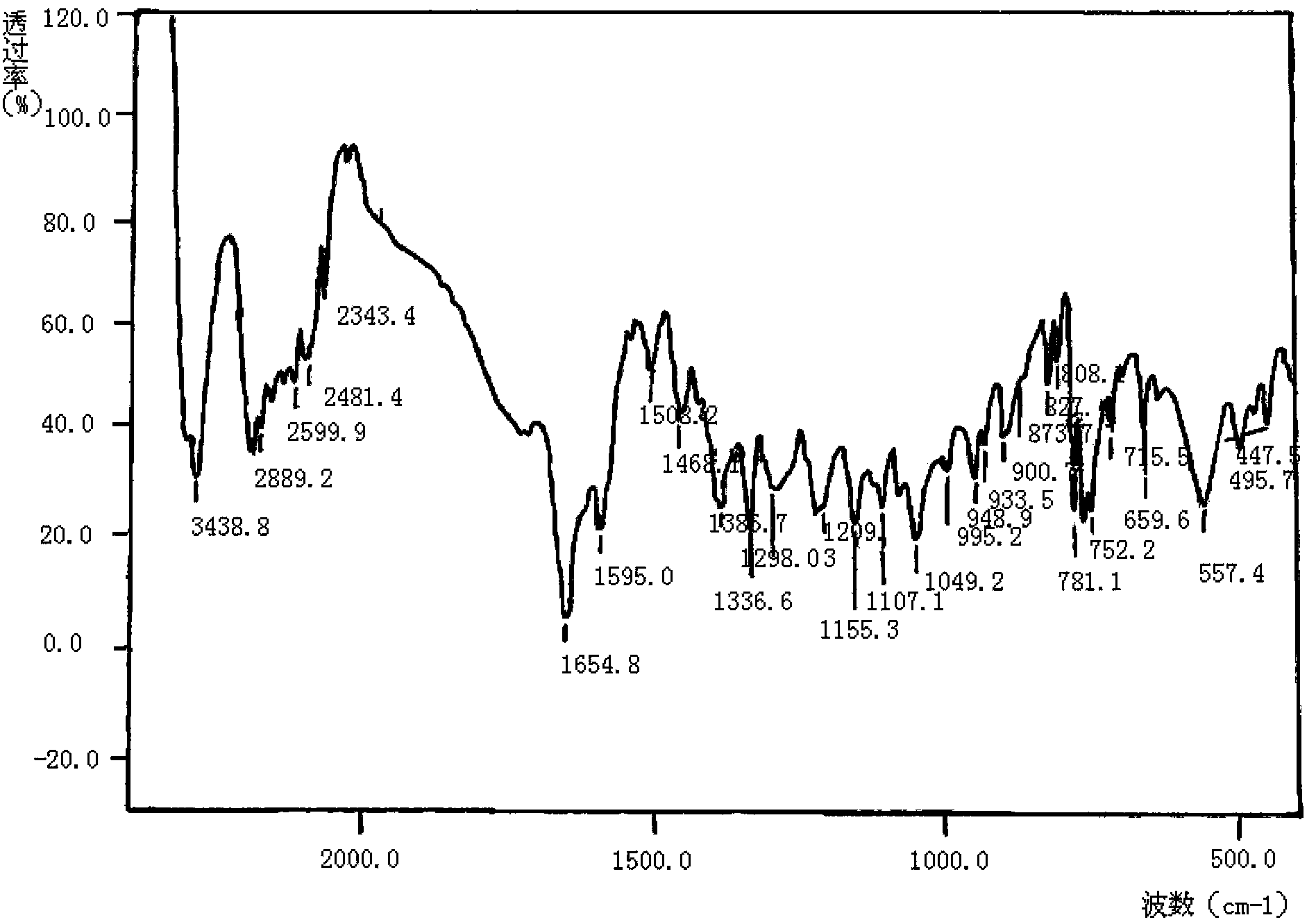
[0093] 1. Determination of the structure of meropenem:
[0094] The test results of the physical properties of the meropenem raw material are as follows:
[0095] Elemental Analysis: C 17 h 25 N 3 o 5 S·3H 2 o
[0096] Calculated: C, 46.67%; H, 7.14%; N, 9.60%; S, 7.33%;
[0097] Found: C, 46.46%; H, ...
Embodiment 3
[0131] Add 12kg of crude meropenem to 180kg of water, stir to form a suspension, raise the temperature of the feed solution to 80°C to dissolve it, and then lower the temperature to 20°C.
[0132] Add 240g of activated carbon for decolorization, and filter.
[0133] Add 360 L of crystallization solvent acetone to the filtrate, and continue to stir and grow the crystal for 3 hours after the addition is complete.
[0134] Filter, wash the crystals with acetone, and dry to obtain 10.44 kg of the first refined meropenem.
[0135] The quality of the first refined meropenem: the total amount of impurities is 0.62%; the residual organic solvent is 0.25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com