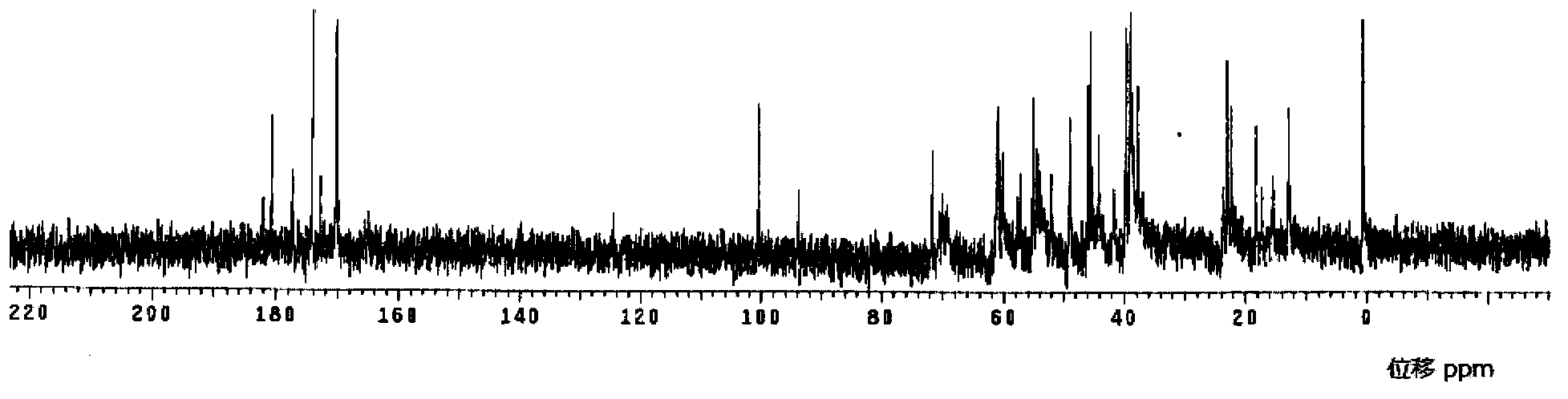
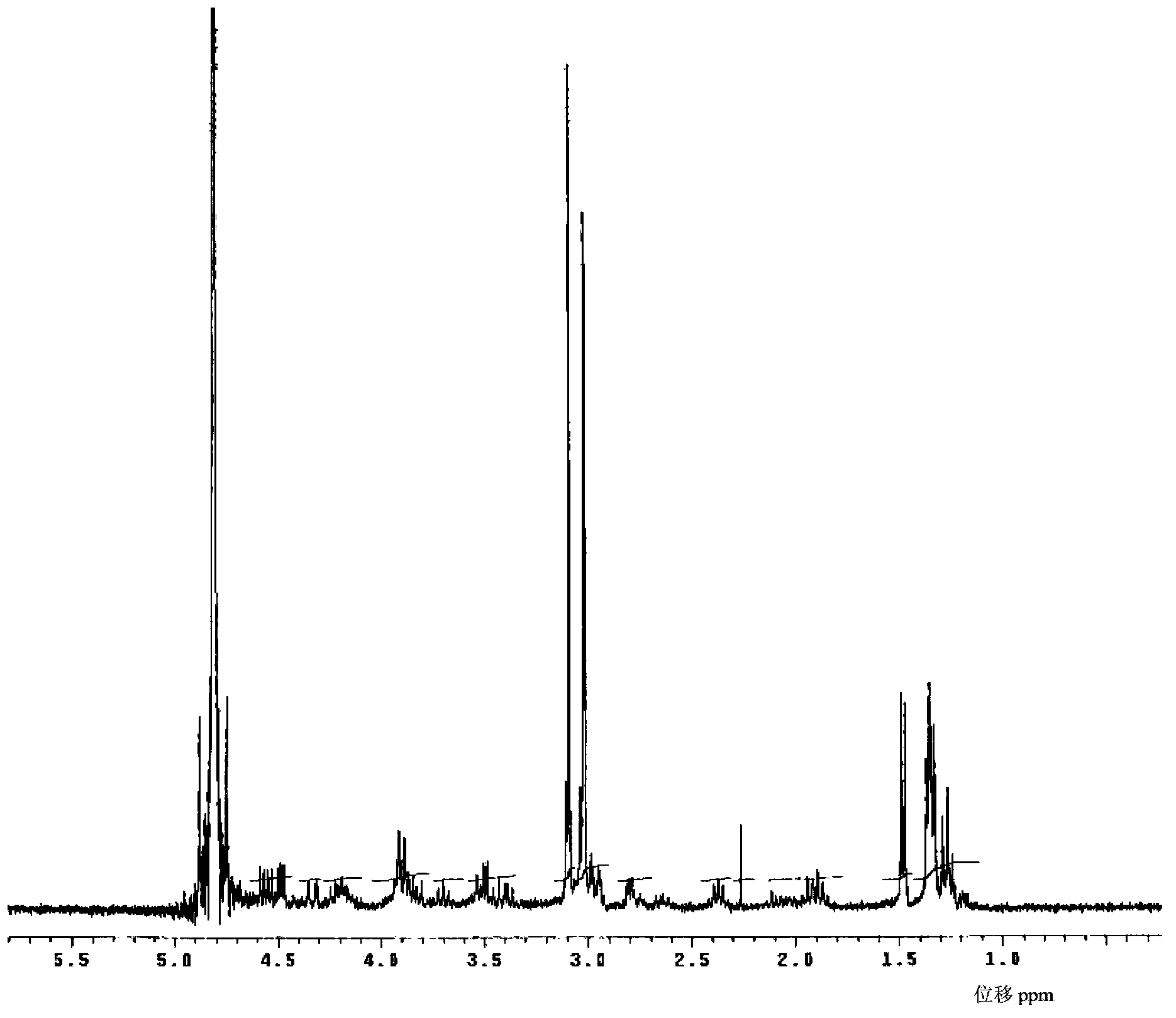
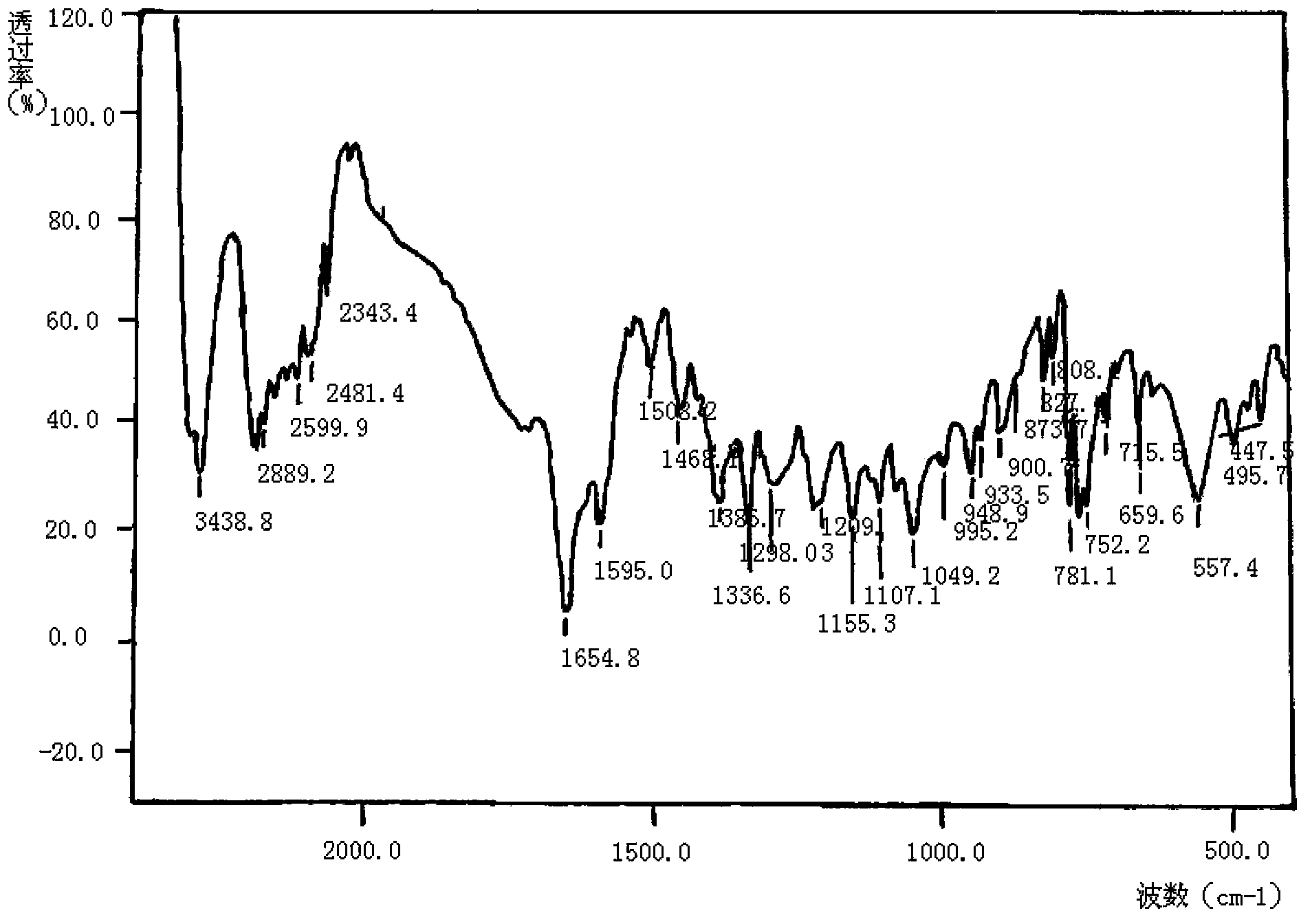
Meropenem raw medicine, preparation method thereof and pharmaceutical composition containing same
A technology for meropenem and APIs, applied in pharmaceutical formulations, drug delivery, antibacterial drugs, etc., can solve the problems of clogging of nanofiltration membranes, difficulty in obtaining organic solvents, and high concentrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Add 1 kg of crude meropenem to 20 kg of water for injection at a temperature of 10°C, stir and mix evenly, heat the suspension to 80°C through a heat exchanger to dissolve it, and quickly drop it to room temperature 25°C through a heat exchanger to obtain a solution I.
[0113] The decolorized solution I was filtered in-line through an activated carbon filter to obtain the filtrate II.
[0114] Release 210 g of filtrate II, set the temperature to 10° C., add 1050 ml of acetone, stir for 15 minutes, and then filter to obtain seed crystals. Cool down to 2°C, add the prepared seed crystals to the remaining 20.8 kg of filtrate II, keep the stirring speed at 130 rpm, grow the crystals for 8 hours, and filter to obtain mother liquor 3, which is then concentrated by nanofiltration in the next step.
[0115] The filter cake was rinsed with acetone, dried under vacuum at 30°C for 5 hours, and pulverized to obtain 680 g of meropenem raw material with a yield of 68%.
[0116] Am...
Embodiment 2
[0155] Put 21.0 kg of the refined mother liquor 3 of meropenem obtained in Example 1 into the solution tank of the nanofiltration equipment; control the outlet pressure of the nanofiltration equipment to 18×10 5 Pa began to concentrate, and the temperature of the feed liquid was controlled at 10°C to 25°C during the concentration process. It took 98 minutes, and the concentrate was reduced from 21.0kg to 2.70kg, and 18.30kg of concentrated wastewater was obtained. The concentrated wastewater was sampled to detect the content of meropenem, and the result was undetected. The concentrated solution was taken to detect that the content of meropenem was 10%.
[0156] Add 2.70kg of the concentrated solution to 152L of the filtered mother liquor (mother liquor 2) where the crude product has been precipitated, and stir at a temperature of 10-15°C for 30 minutes; filter, wash crystals, and dry to obtain 186g of crude meropenem; take a sample to detect the crude meropenem Impurities and ...
Embodiment 3
[0159] Add 20kg of crude meropenem to 300kg of water for injection at a temperature of 20°C, stir and mix evenly, heat the suspension to 70°C through a heat exchanger to dissolve, and quickly drop it to 20°C through a heat exchanger to obtain solution I .
[0160] The decolorized solution I was filtered in-line through an activated carbon filter to obtain the filtrate II.
[0161] Release 6.4kg of filtrate II, set the temperature at 5°C, add 25.6L of tetrahydrofuran, stir for 20 minutes, and then filter to obtain seed crystals. Cool down to 0°C, add the prepared seed crystals to the remaining 288 kg of filtrate II, keep the stirring speed at 120 rpm, grow the crystals for 10 hours, and filter to obtain mother liquor 3, which is then concentrated by nanofiltration in the next step.
[0162] The filter cake was rinsed with acetone, dried under vacuum at 25° C. for 6 hours, and pulverized to obtain 14 kg of meropenem raw material with a yield of 70%.
[0163] Among them, the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com