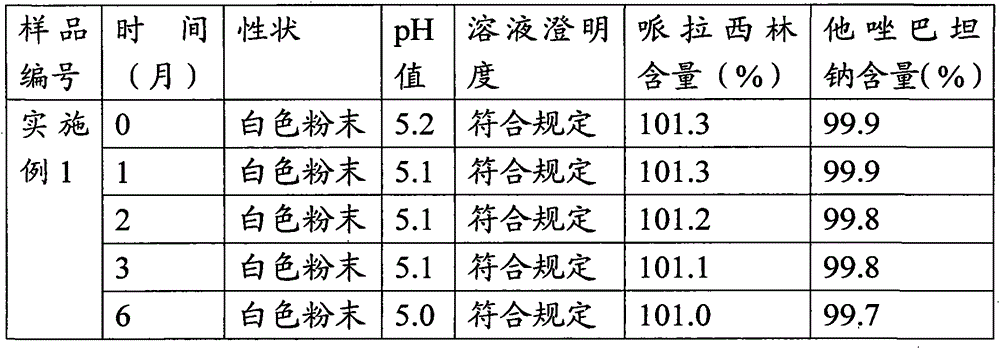
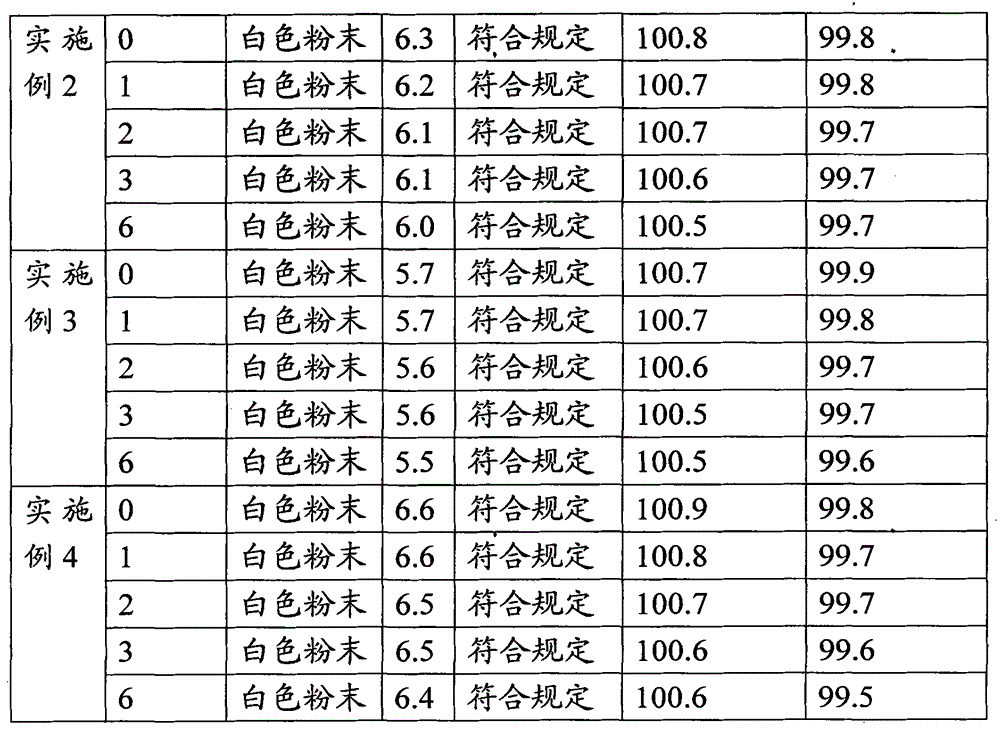
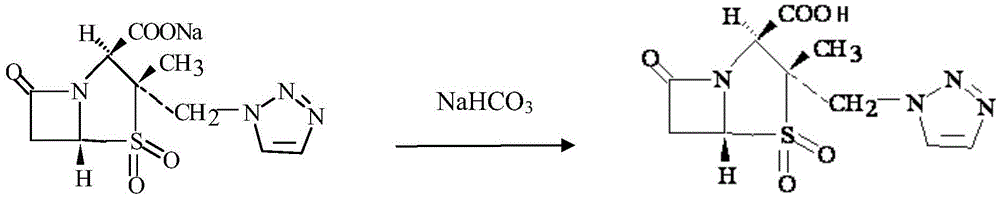
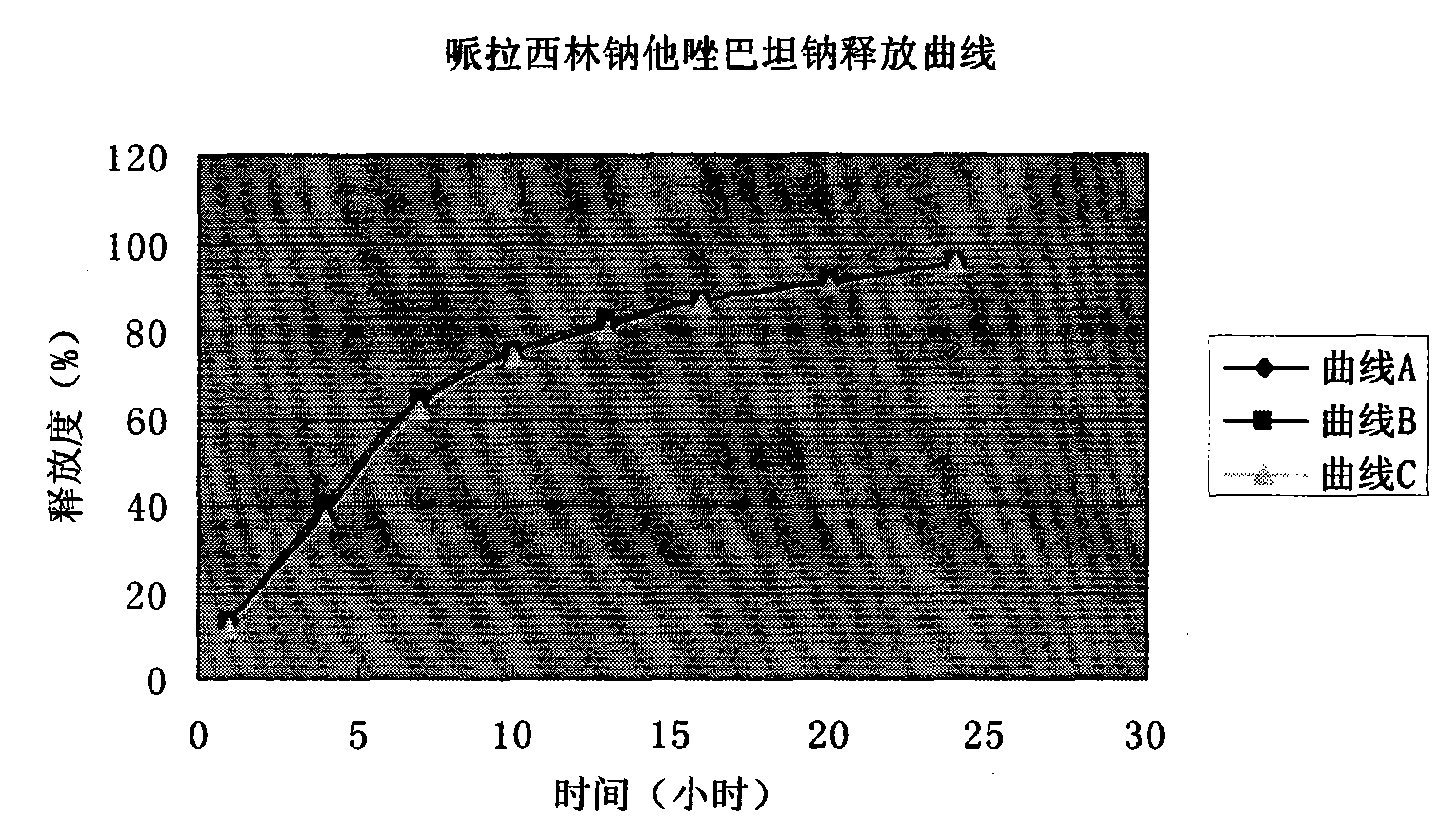
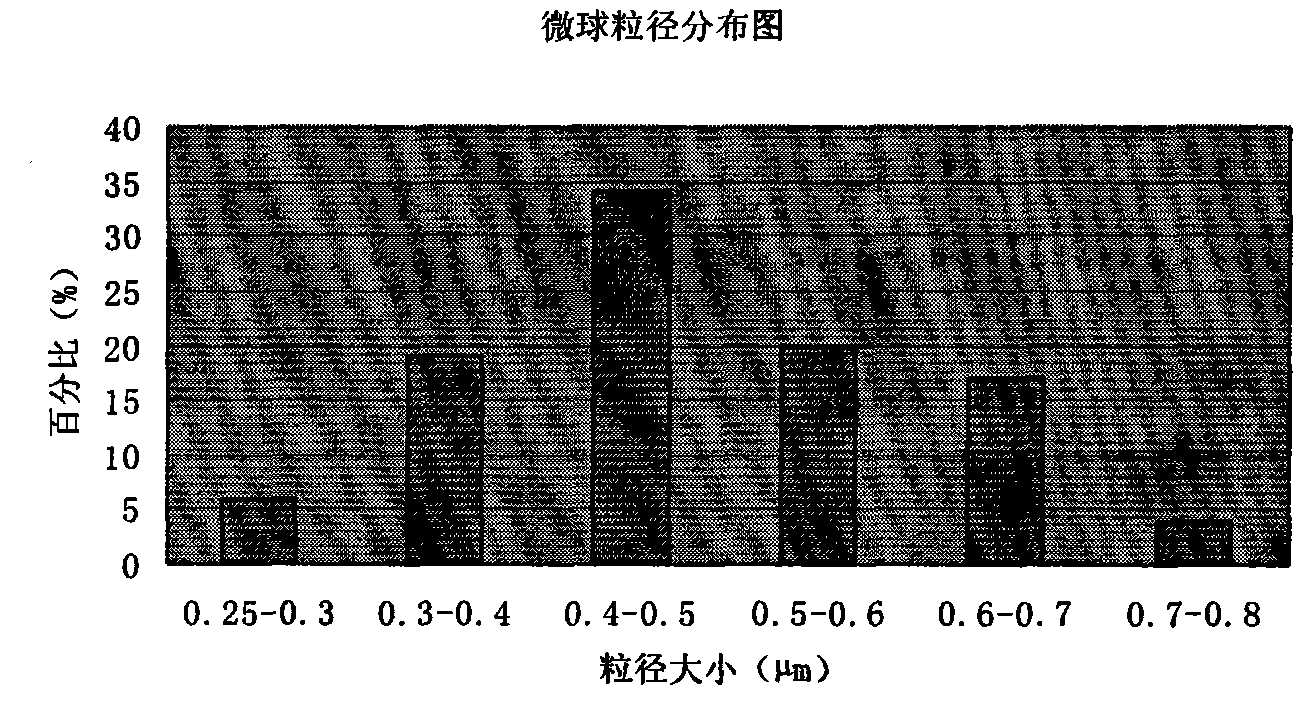
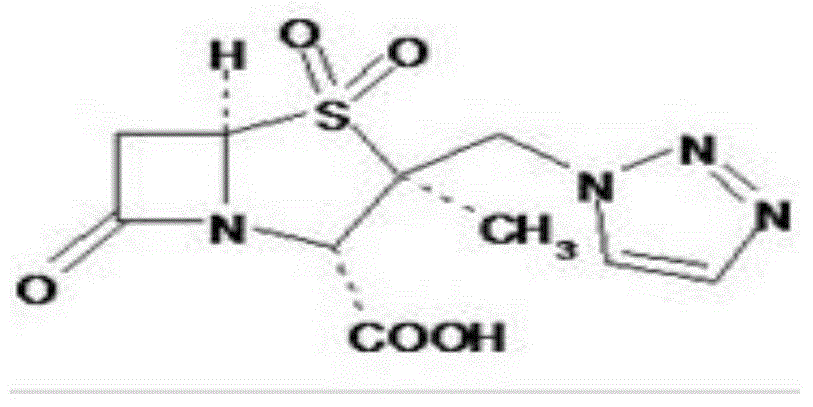
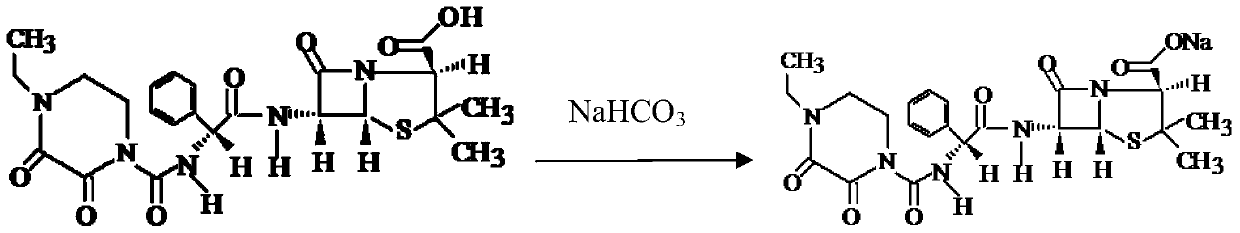
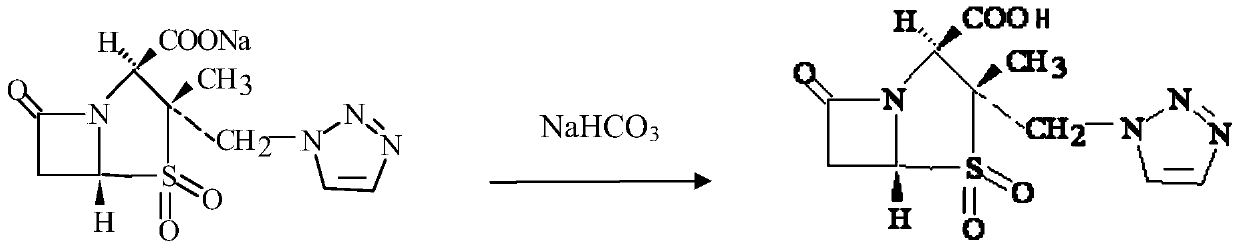
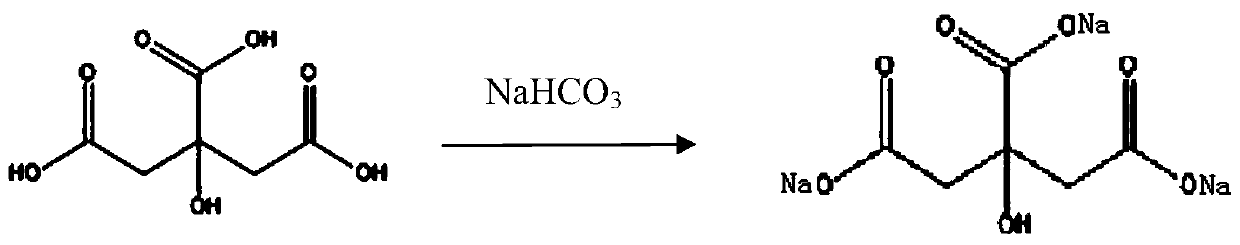
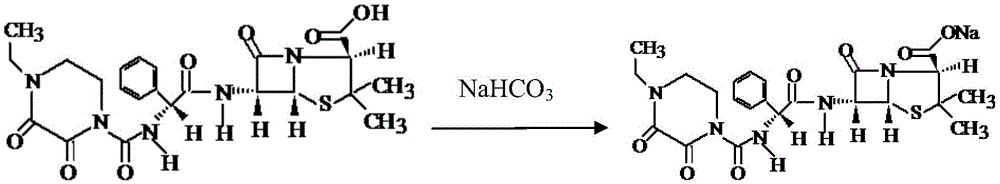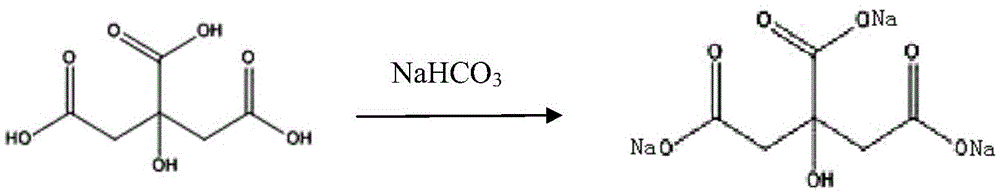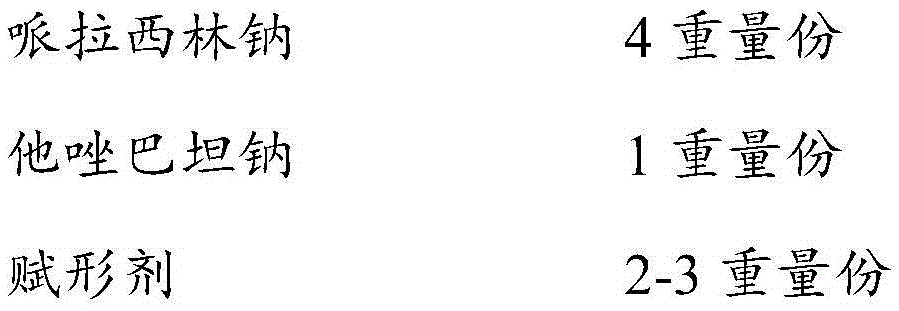Patents
Literature
38 results about "Piperacillin Sodium/ Tazobactam Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosing: Pediatric. Note: Piperacillin and tazobactam is a combination product; each 3.375 g vial contains 3 g piperacillin sodium and 0.375 g tazobactam sodium in an 8:1 ratio. Dosage recommendations in pediatric patients are based on the piperacillin component.
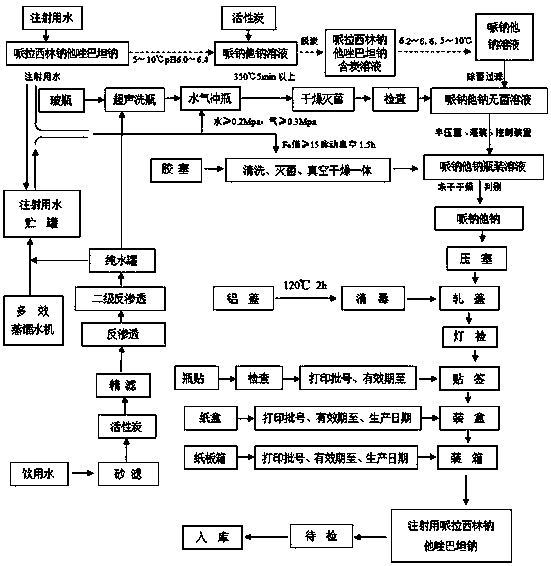
Piperacillin sodium and tazobactam sodium compound preparation for injection
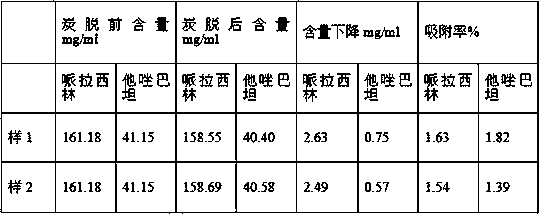
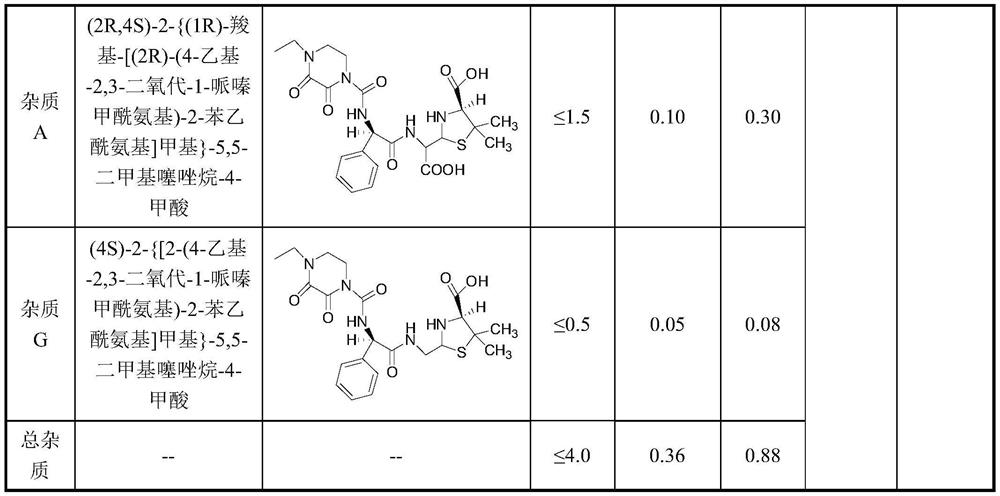
InactiveCN1732930AGood treatment effectStrong mixed infectionAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumOtolaryngology/ENT
The invention discloses a compound preparation of piperacillin sodium and tazobactam sodium for injection, which comprises piperacillin sodium and tazobactam sodium by the weight ratio of 3-4:0.8-1.2. The preparation can be used for treating respiratory system infection, urological infection, otologia infection and skin infection.
Owner:NORTH CHINA PHARMA GROUP CORP
Suspension powder injection of piperacillin sodium and tazobactam sodium pharmaceutical composition and new application thereof
InactiveCN101632670AAvoid it happening againImprove stabilityPowder deliveryLyophilised deliveryPiperacillin Sodium/ Tazobactam SodiumPharmacology
The invention discloses a suspension powder injection of a piperacillin sodium and tazobactam sodium pharmaceutical composition, and further discloses an application thereof to preparing medicines for treating lung abscess.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Production method of piperacillin sodium tazobactam sodium freeze-drying preparation for injection
ActiveCN103239454AEasy to judgeQuick responsePowder deliveryAntiinfectivesHigh concentrationSodium bicarbonate
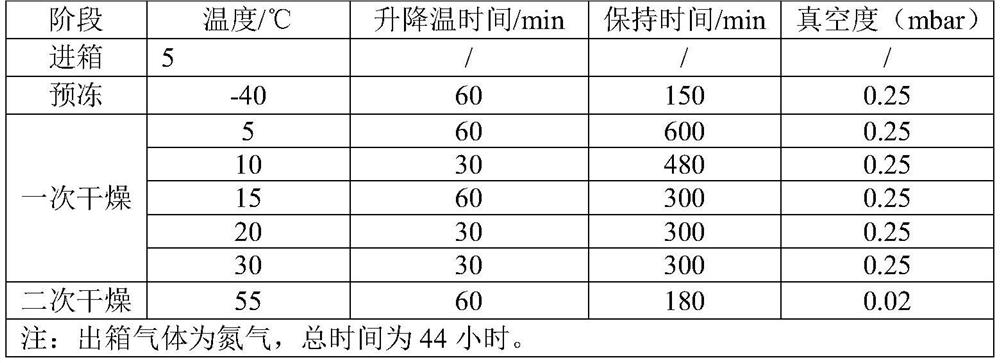
The invention discloses a production method of a piperacillin sodium tazobactam sodium freeze-drying preparation for injection and belongs to the technical field of medicines. The production method comprises the steps of reacting, prefreezing, sublimation drying, drying again and taking out of a box. According to the production method, in the step of reacting, sodium hydroxide is adopted for substituting sodium bicarbonate, so that no bubble is produced in a reaction process; and a sodium hydroxide solution is dropwise added into a turbid liquid containing piperacillin acid and tazobactam acid, reaction is carried out in a manner of adding nitrogen to the bottom of a reaction tank, and reaction speed of feed liquid can be increased while time that local concentration of sodium hydroxide is overhigh can be shortened, so that degradation effect of a high-concentration sodium hydroxide solution on piperacillin sodium tazobactam sodium is effectively avoided. According to the production method, mutual combination between quick freezing and slow cooling is realized on prefreezing of the piperacillin sodium tazobactam sodium freeze-drying preparation, so that a crystal form of the obtained piperacillin sodium tazobactam sodium freeze-drying preparation product, insoluble particles and related impurities of freeze-drying products are at the best level. The product piperacillin sodium tazobactam sodium freeze-drying preparation for injection prepared by using the production method disclosed by the invention is good in quality and low in impurity content.
Owner:山东安信制药有限公司
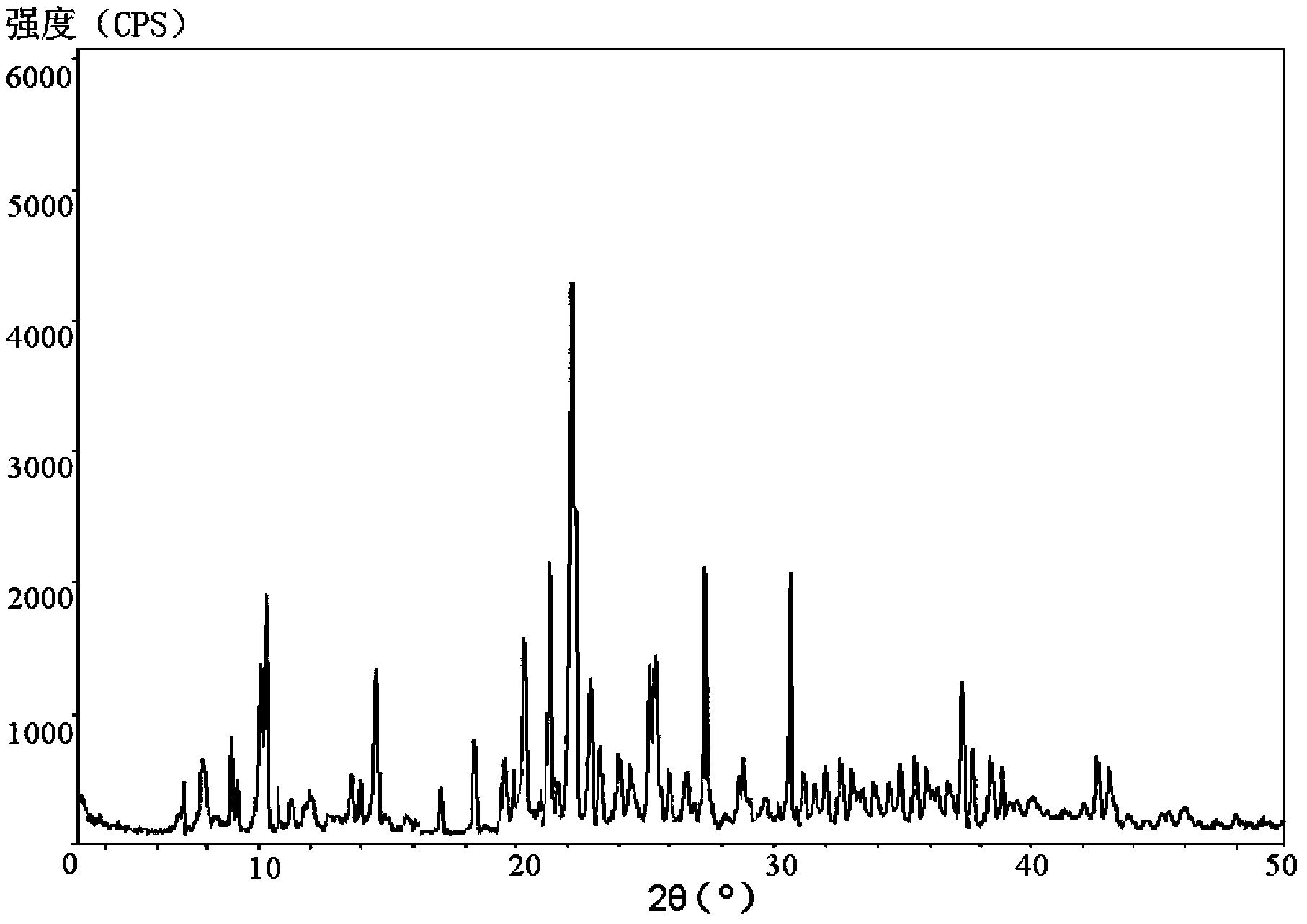
Piperacillin sodium-tazobactam sodium medicine composition and preparation method thereof
ActiveCN103340866ALow polymer contentImprove stabilityAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumMass ratio
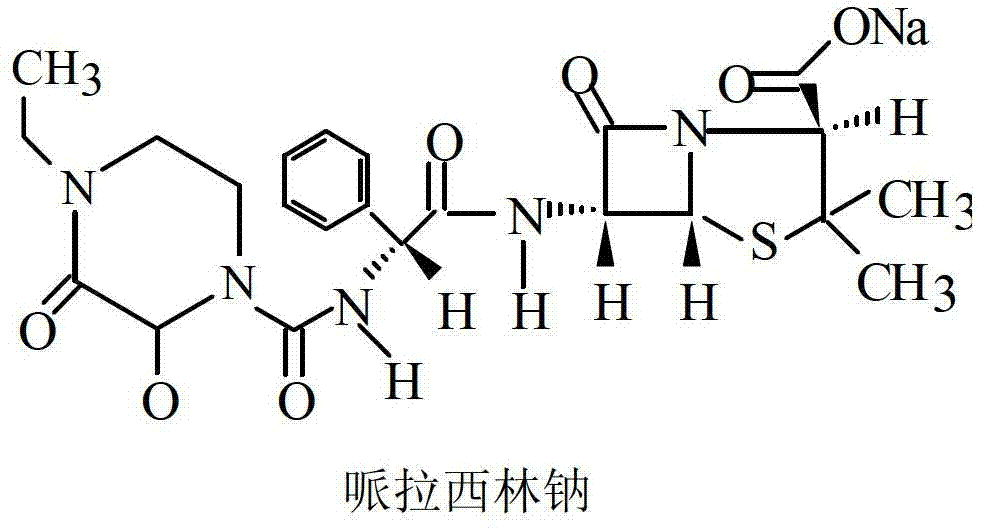
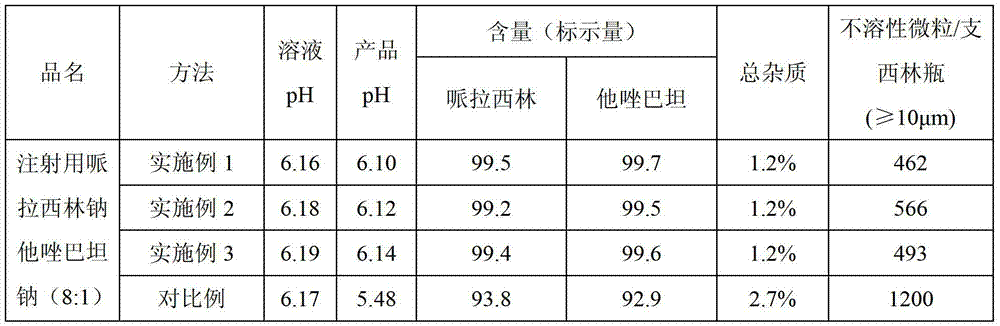
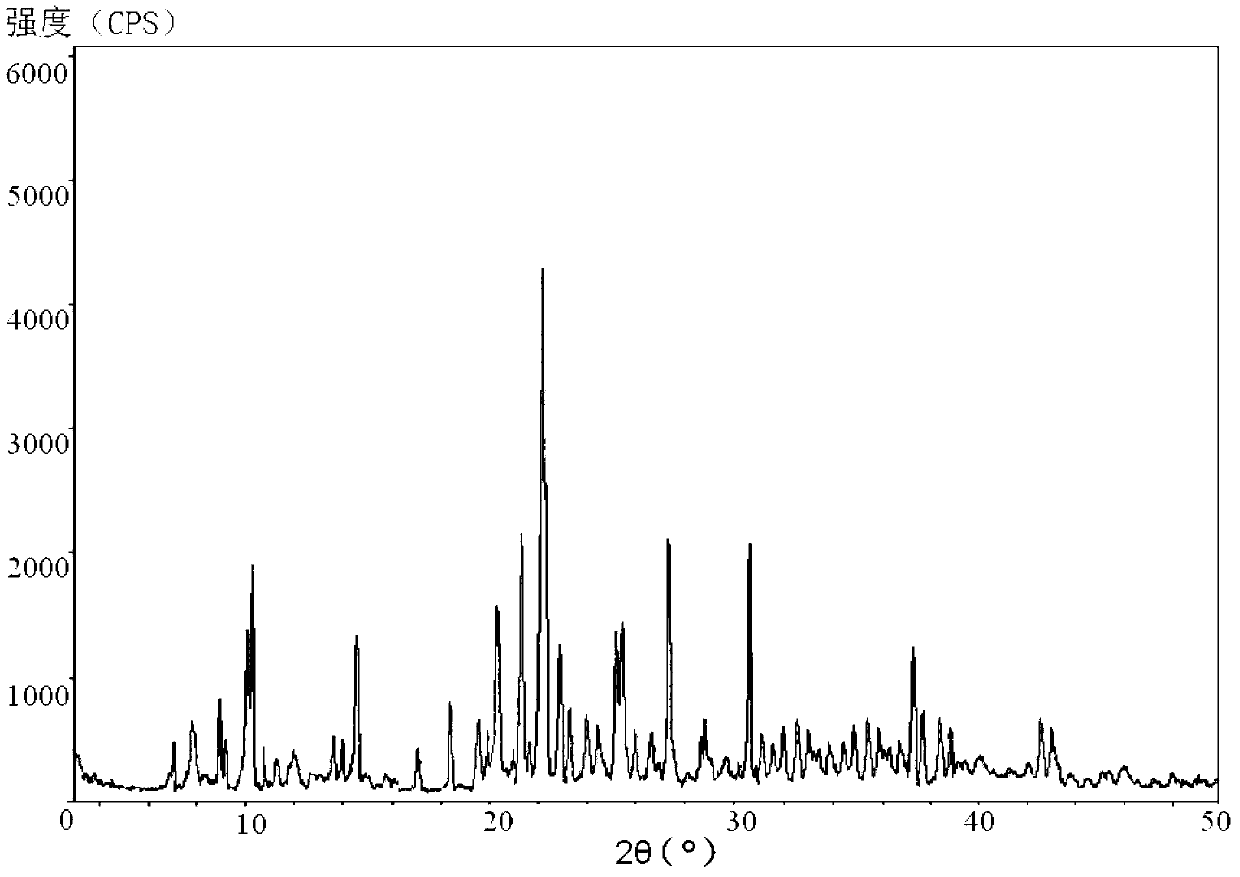
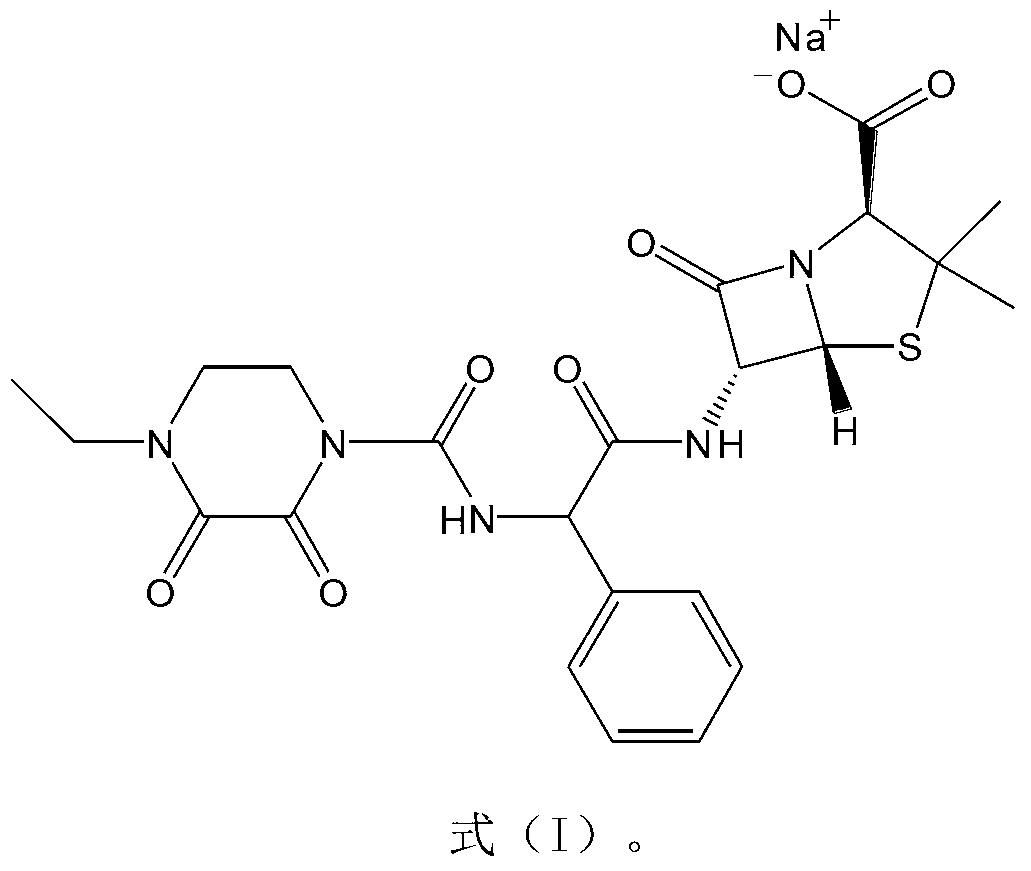
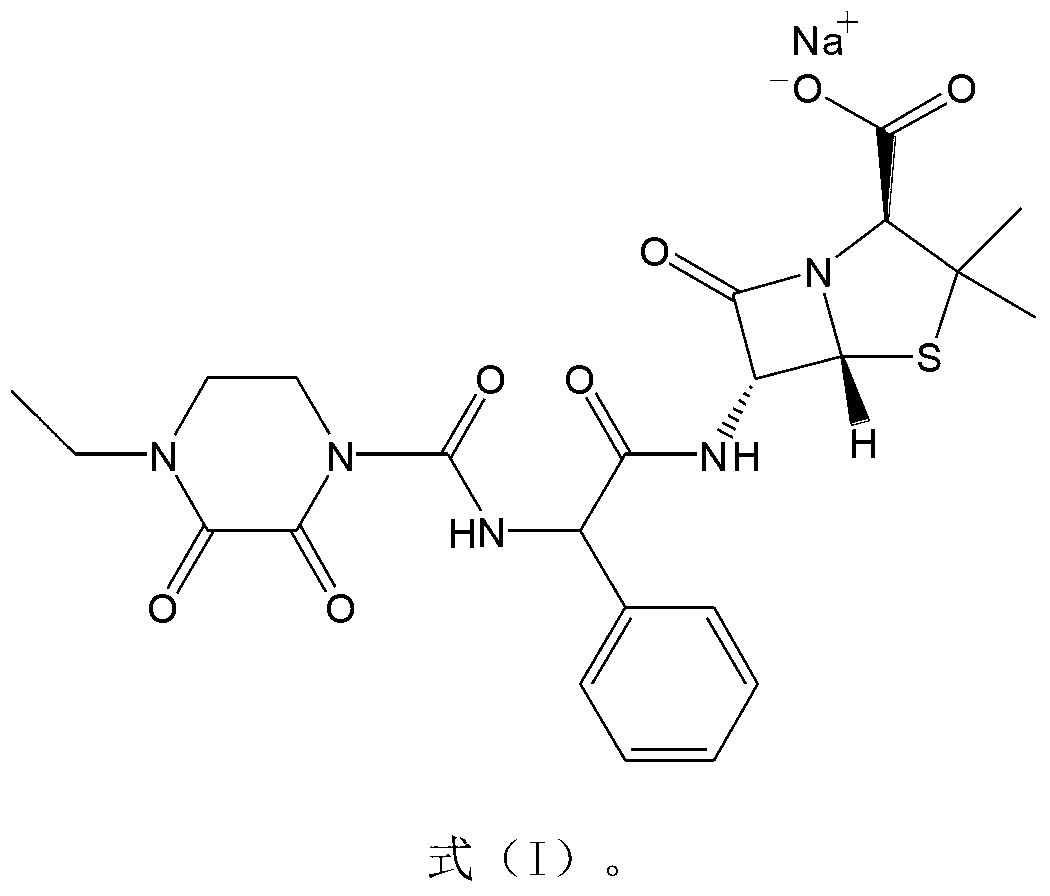
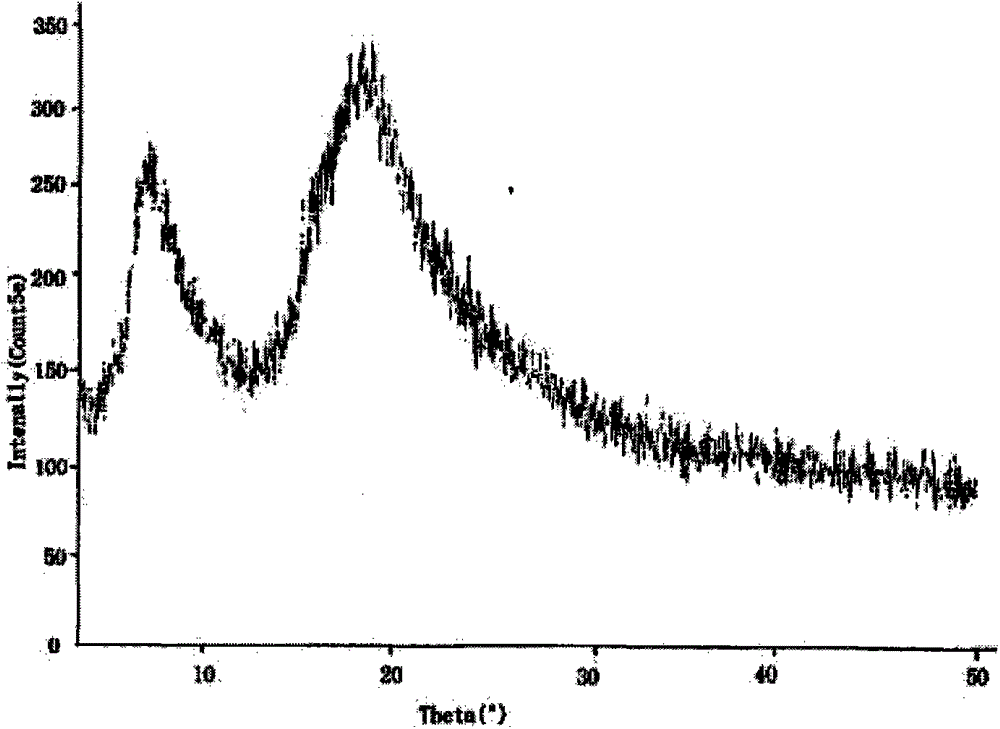
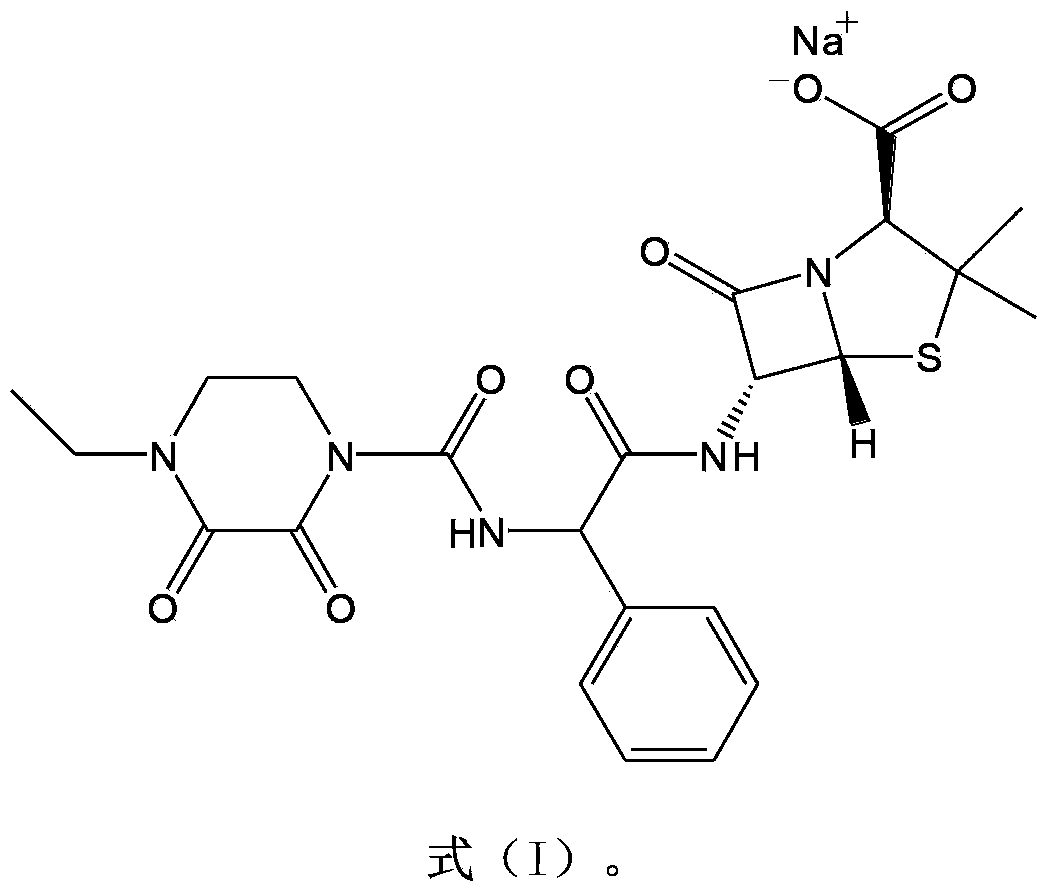
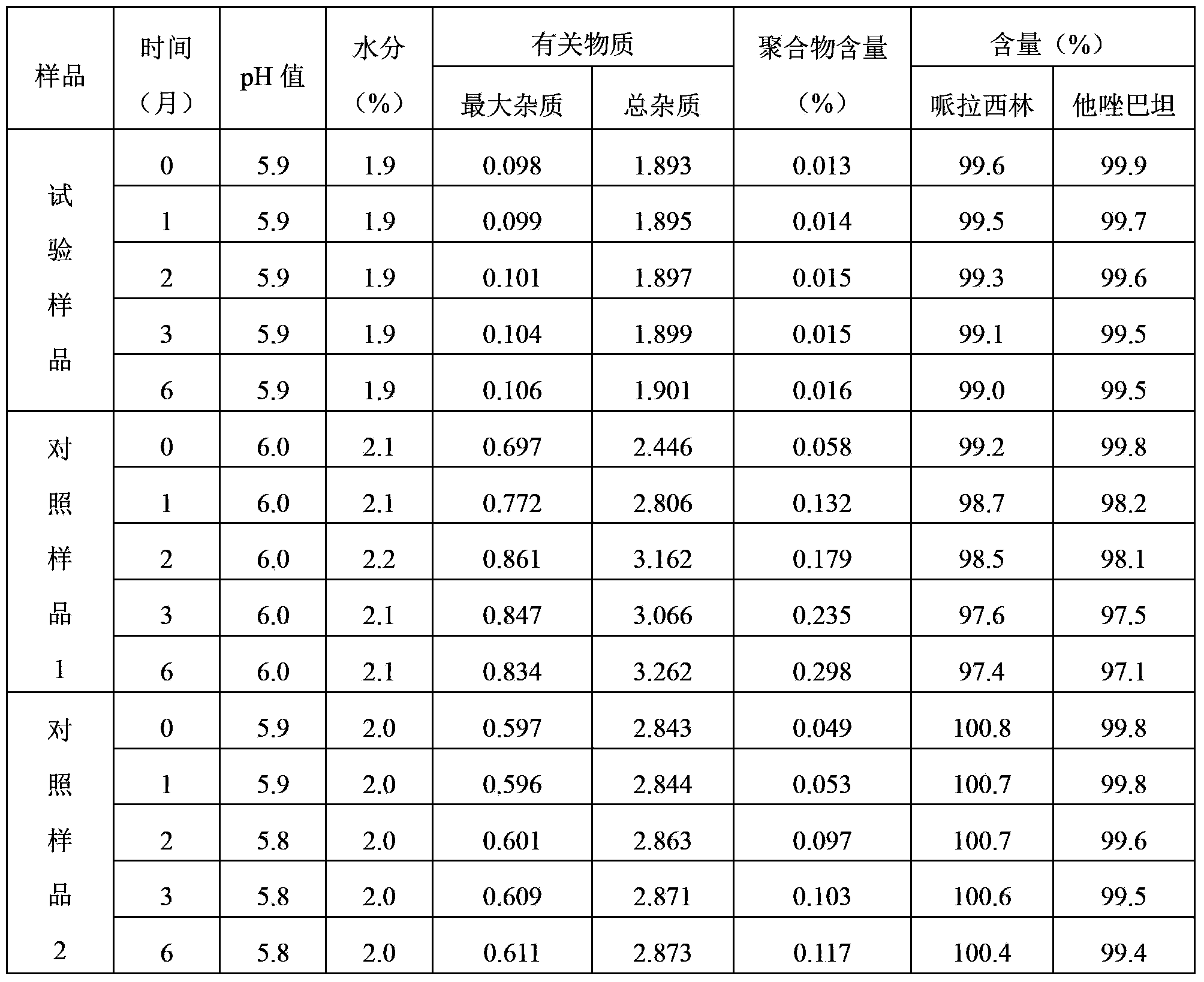
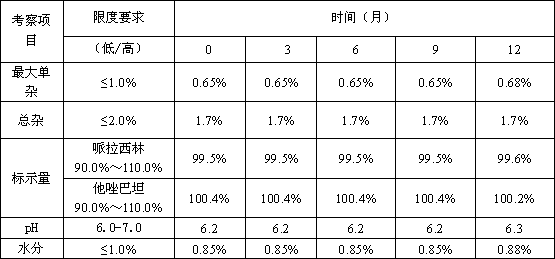
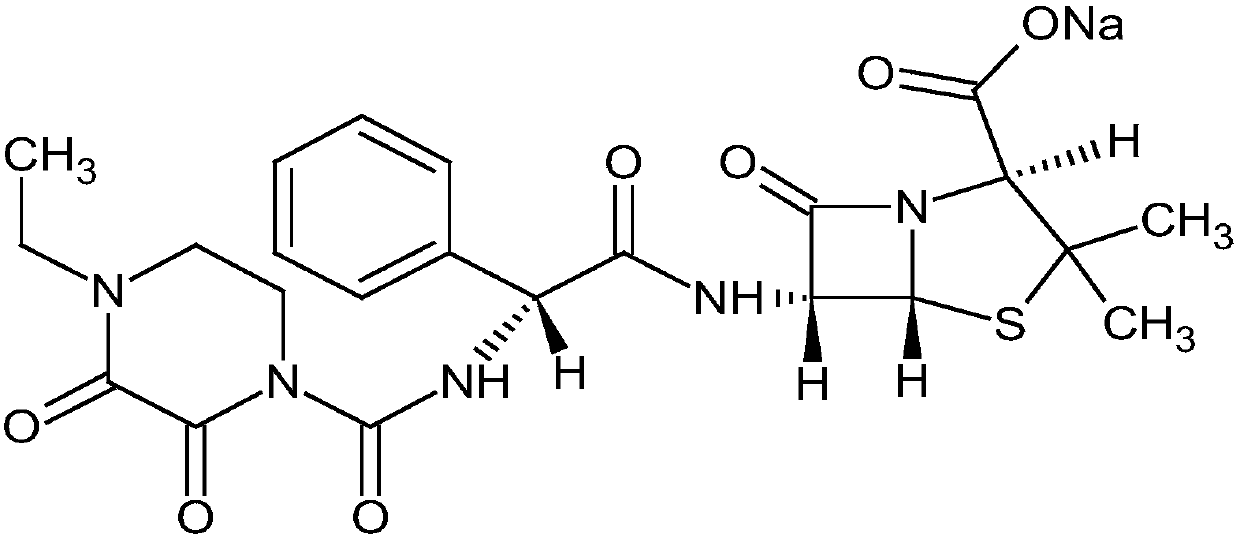
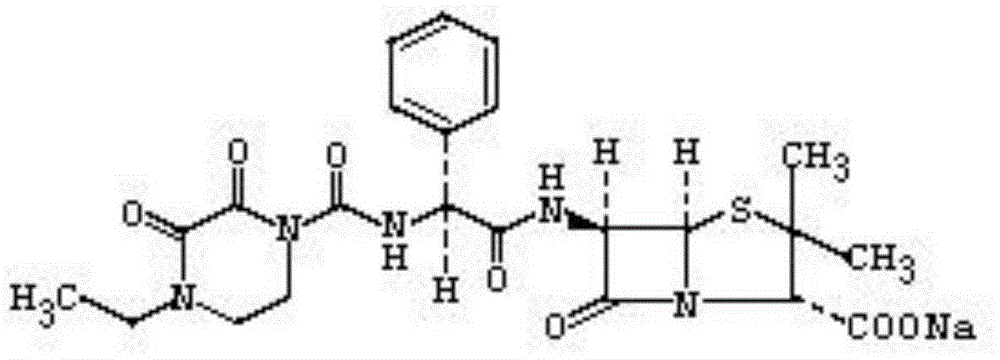
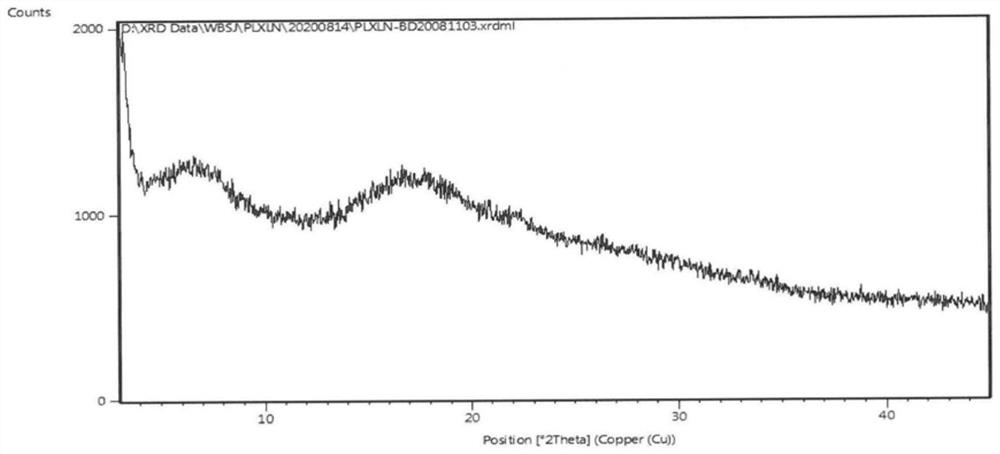
The invention belongs to the technical field of medicine, and in particular relates to a piperacillin sodium-tazobactam sodium medicine composition and a preparation method of the medicine composition. The medicine composition is a sterile powder injection; the mass ratio of the piperacillin sodium to the tazobactam sodium in the medicine composition is 4-20:1; the X-ray powder diffraction pattern of the piperacillin sodium measured by means of powder X-ray diffraction measurement method is shown in Figure 1; the chemical structural formula of the piperacillin sodium is shown in Formula (I); and the content of the piperacillin sodium polymer in the medicine composition provided by the invention is quite low and is not changed obviously in an accelerated test condition and in a long-term test condition. The medicine composition prepared from the piperacillin sodium and the tazobactam sodium provided by the invention also has the advantages of low polymer content and good stability.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Liposome injection of pharmaceutical composition comprising piperacillin sodium and tazobactam sodium
InactiveCN101890015ASimple preparation processFacilitated releaseAntibacterial agentsLiposomal deliveryPiperacillin Sodium/ Tazobactam SodiumLiposome membrane
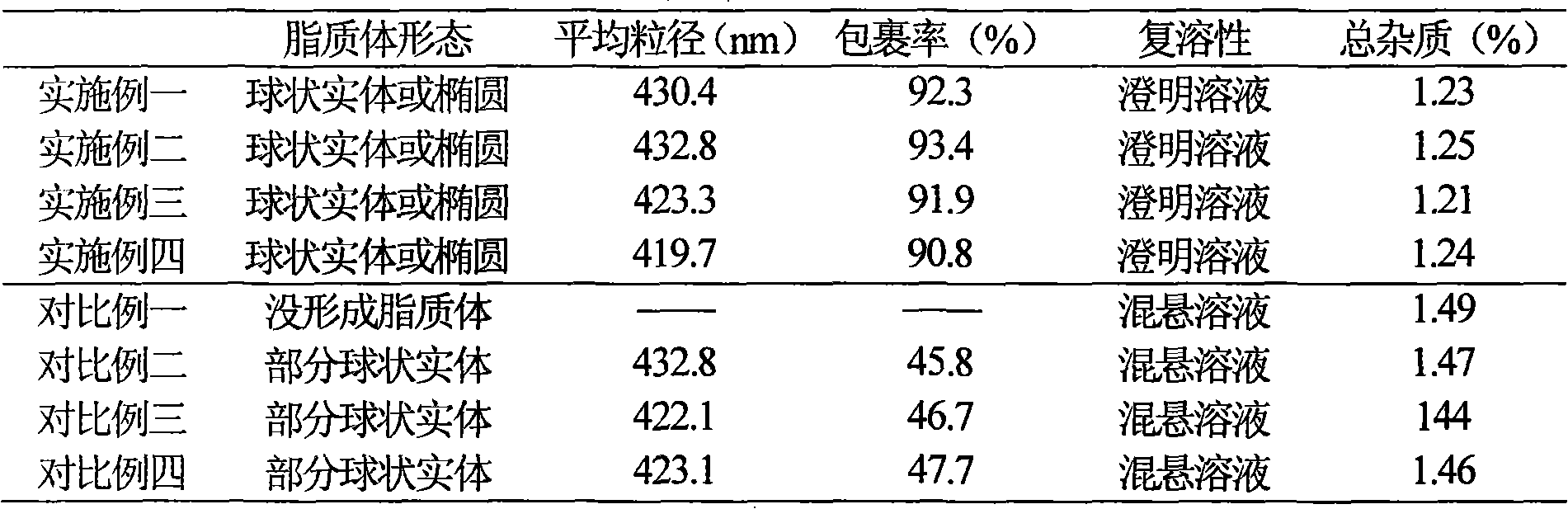
The invention discloses a liposome injection of a pharmaceutical composition comprising piperacillin sodium and tazobactam sodium. The liposome injection mainly comprises the following components in part by weight: 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4.5 to 13.5 parts of liposome membrane material and membrane material additive, 0.9 to 1.8 parts of frozen dry excipient and 0.45 to 0.9 part of antioxidant.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Piperacillin sodium and tazobactam sodium pharmaceutical composition and preparation method thereof
ActiveCN103550216AEasy to controlDry fastAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumPowder injection
The invention discloses a piperacillin sodium and tazobactam sodium pharmaceutical composition and a preparation method thereof. The pharmaceutical composition mainly comprises piperacillin sodium and tazobactam sodium, as well as a disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair, and the powder injection is prepared by spraying and drying. The disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair is adopted for playing a role in buffering the pH of a solution, thus ensuring the content stability of piperacillin sodium, and no generation of carbon dioxide; the preparation technology is simple, convenient to control, fast to dry, and applicable to continuous large-scale production. The piperacillin sodium and tazobactam sodium powder injection is fast to dissolve, stable in quality, free from crystallization and degradation products, and the solution clarity meets the specification.
Owner:REYOUNG PHARMA
Preparation method of piperacillin sodium and tazobactam sodium for injection
ActiveCN105616415AAvoid decompositionImprove acidity stabilityAntibacterial agentsPowder deliverySodium bicarbonatePiperacillin Sodium/ Tazobactam Sodium
The invention discloses a preparation method of piperacillin sodium and tazobactam sodium for injection. The preparation method comprises the following steps: adding water into a blending tank, cooling to 5-10 DEG C, adding citric acid, then slowly adding sodium bicarbonate, and after reacting, reading the pH value and adjusting the pH value to be 6.3-6.6; adding piperacillin acid into the blending tank, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, adding tazobactam acid, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, carrying out vacuum removal on carbon dioxide gas by suction, and after the feed liquid in the blending tank is stabilized, reading the pH value and adjusting the pH value to be 6.0-6.5; and carrying out aseptic filtration and freeze-drying to obtain raw powder of piperacillin sodium and tazobactam sodium for injection. According to the preparation method, by adding salified citric acid, the acidity stability of the product is increased, the decomposition of piperacillin sodium and tazobactam sodium is inhibited, and the storage stability of the product is increased.
Owner:山东安信制药有限公司
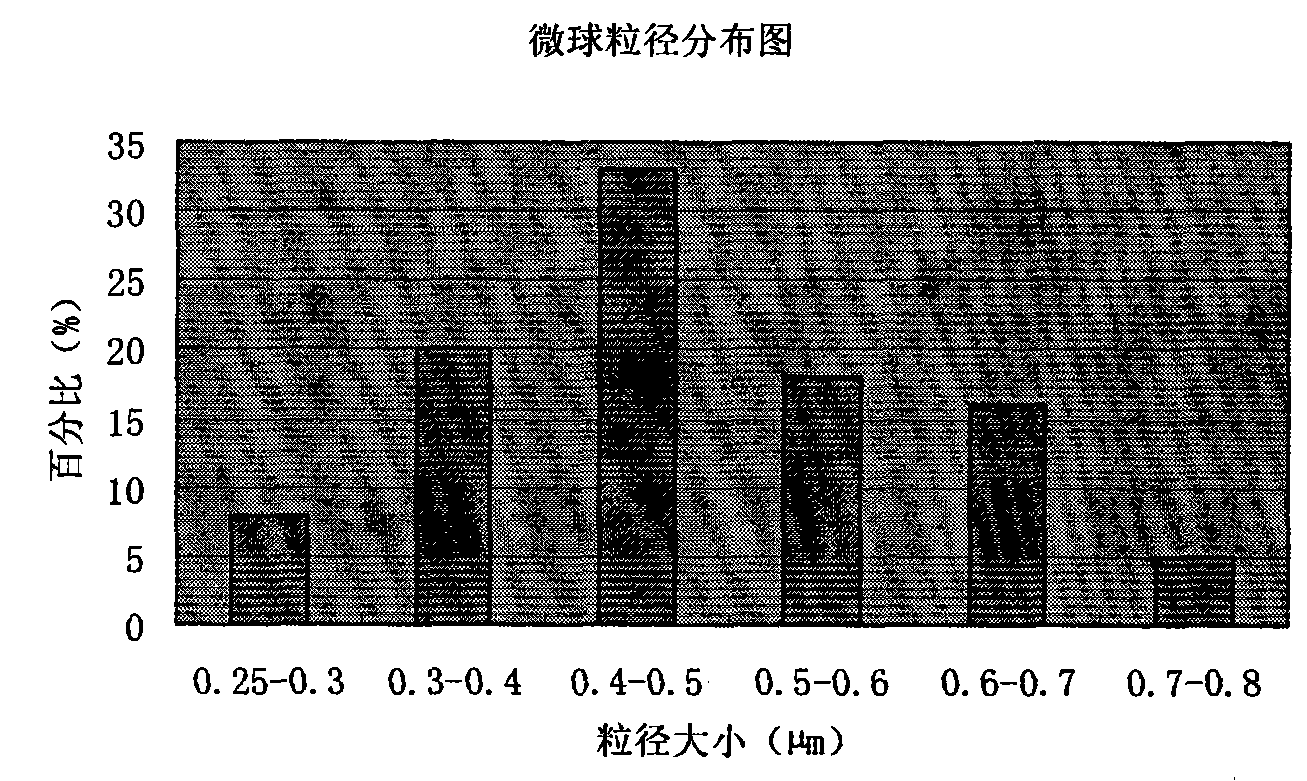
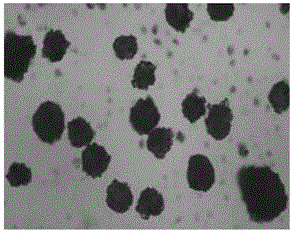
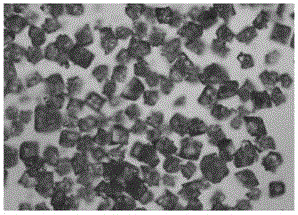
Piperacillin sodium-tazobactam sodium medicinal composition microsphere injection
InactiveCN101890016AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryPiperacillin Sodium/ Tazobactam SodiumMANNITOL/SORBITOL
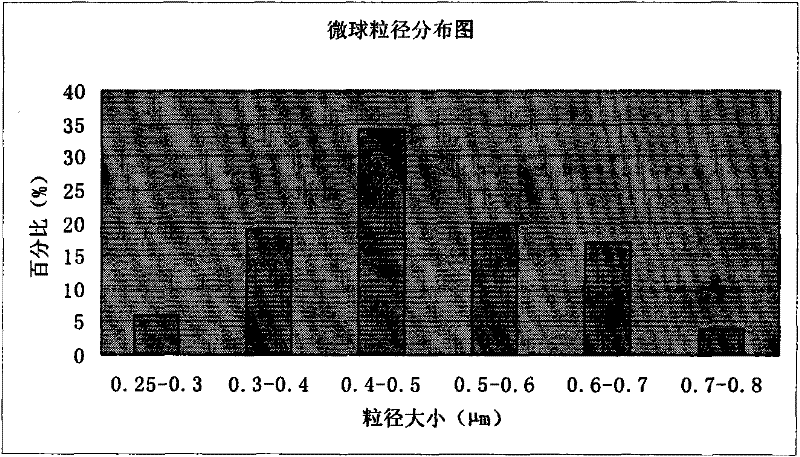
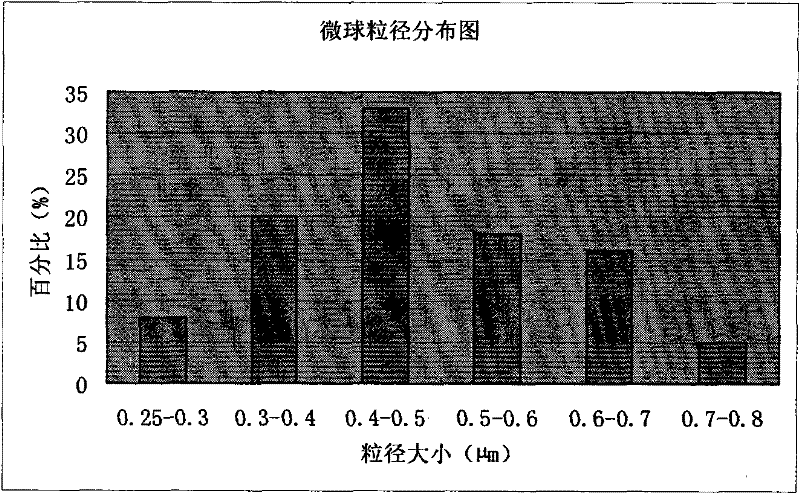
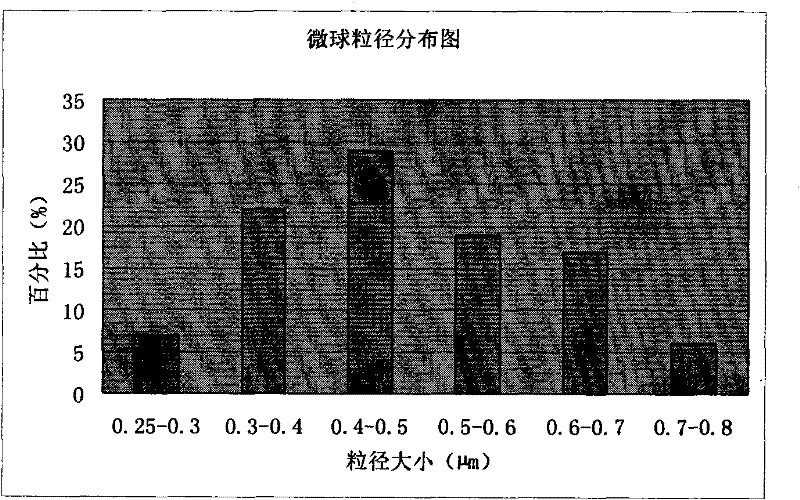
The invention discloses piperacillin sodium-tazobactam sodium medicinal composition microsphere injection. The injection is characterized by consisting of piperacillin sodium, tazobactam sodium, polylactic acid-polyethylene glycol (PLA-PEG), PEG600, polysorbate 80 and mannitol and particularly consisting of 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4 to 9 parts of PLA-PEG, 6 to 10 parts of PEG600, 1 to 3 parts of polysorbate 80 and 2 to 4 parts of mannitol. Compared with the prior art, the piperacillin sodium-tazobactam sodium medicinal composition microsphere injection prepared by the invention has the characteristics of good stability, high entrapment rate, high preparation technology repeatability, suitability for industrial production, uniform particle distribution, few solvent residue, good injectability and excellent sustained release property.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Piperacillin sodium tazobactam sodium preparation for injection and preparation method thereof
ActiveCN104644637AImprove securityEasy to decompose and removeAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumImpurity
The invention discloses a medicine composition of piperacillin sodium tazobactam sodium and a preparation method thereof. The medicine composition is sterile powder injection, wherein the weight ratio of piperacillin sodium tazobactam sodium is (2-4):1. The piperacillin sodium tazobactam sodium sterile powder injection prepared in the invention has high stability and less impurity; by utilizing the sterile powder injection, the safety and effectiveness of clinical medication are improved greatly.
Owner:NORTH CHINA PHARMA COMPANY +2
Piperacillin sodium and tazobactam sodium sterile powder injection and preparation method thereof
ActiveCN104922126AReduce contentGuaranteed curative effectAntibacterial agentsPowder deliveryVitamin CFiltration
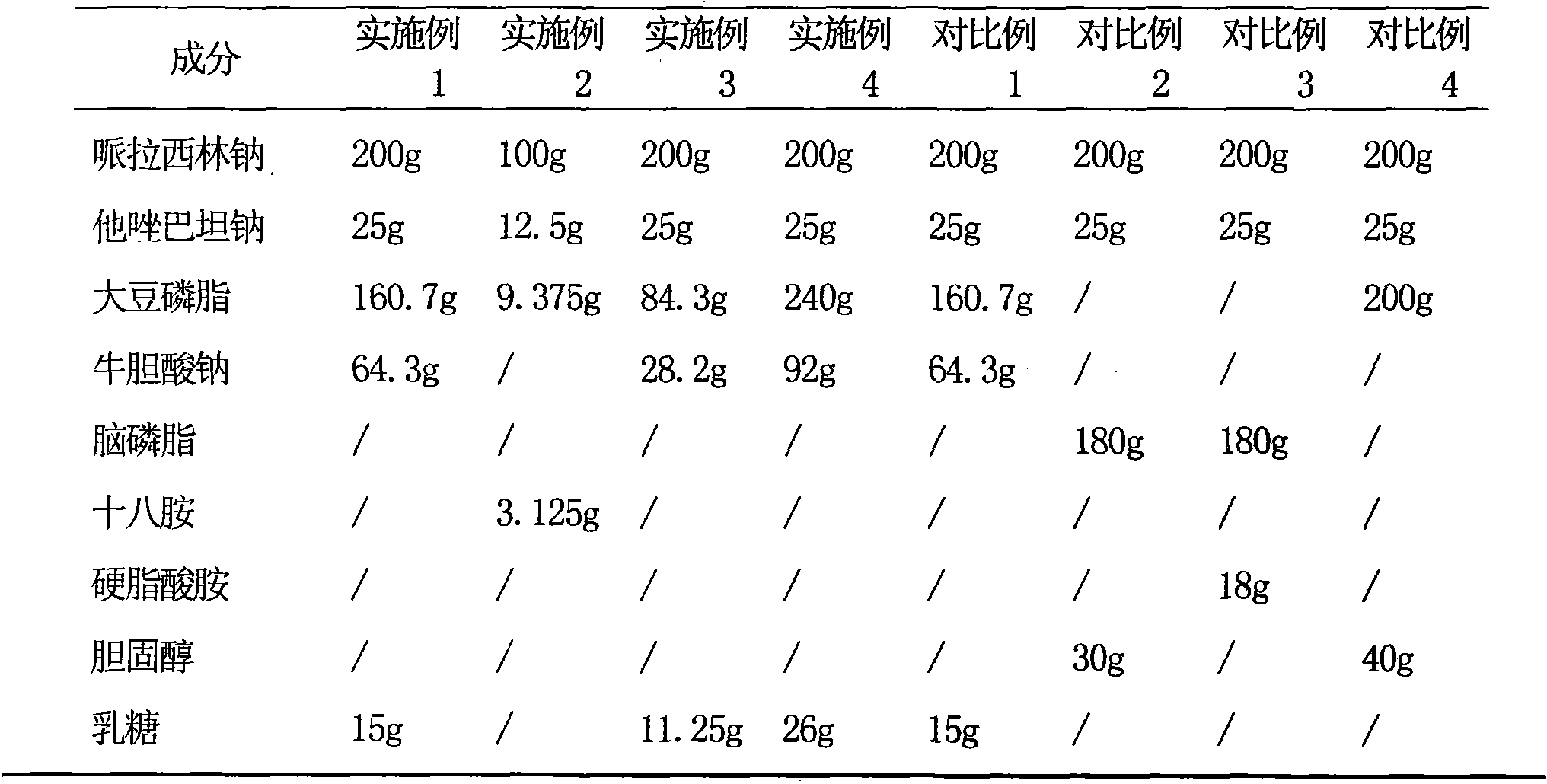
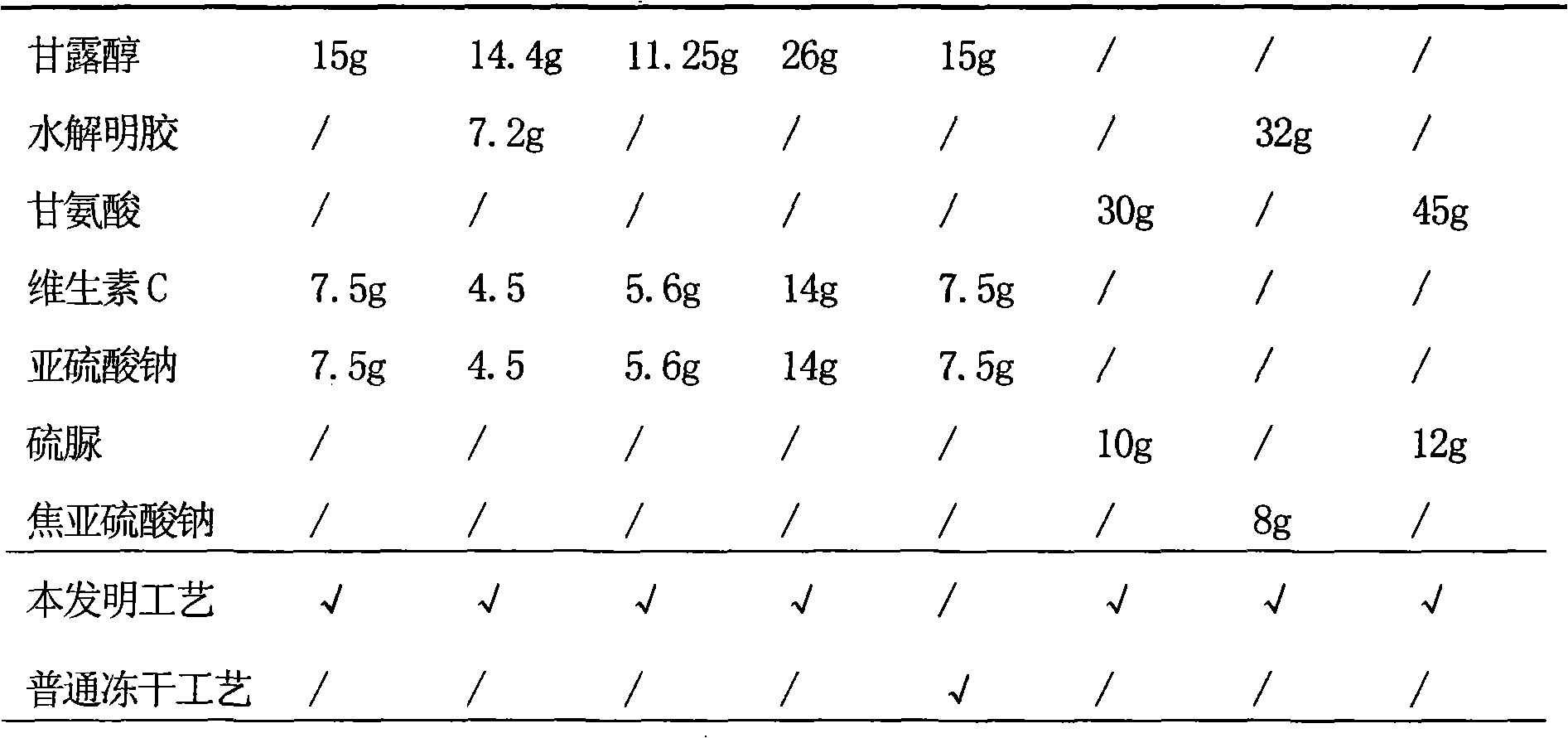
The invention provides a piperacillin sodium and tazobactam sodium sterile powder injection, which comprises the following components: 6 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 40 to 80 parts of mannitol, 10 to 30 parts of vitamin C, 8 to 15 parts of sodium pyrosulfite and 5 to 10 parts of glycine. A preparation method for the piperacillin sodium and tazobactam sodium sterile powder injection comprises the following steps of dissolving all the components in water for injection under inert gas shielding; adding activated carbon for decoloration, and then, filtering to remove the activated carbon; performing refined filtration with a microporous filtering film of 0.22mum to 0.80mum; performing freeze drying to obtain the piperacillin sodium and tazobactam sodium sterile powder injection. Compared with the prior art, the sterile powder injection has the characteristics that the stability of the sterile powder injection is effectively improved by adopting a stabilizer which consists of the mannitol, the vitamin C, the sodium pyrosulfite and the glycine, piperacillin sodium and tazobactam sodium are basically unchanged in stability during preparation and storage; the polymer content is low, the product is guaranteed to be qualified within the period of validity, and the curative effect and the safety in clinical application are guaranteed; the preparation method is simple, low in cost and suitable for industrial mass production.
Owner:HAINAN GENERAL & KANGLI PHARMA
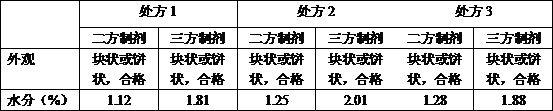
Prescription and technology of piperacillin sodium and tazobactam sodium for injection
InactiveCN108619154AQuality improvementSimple manufacturing process stepsAntibacterial agentsOrganic active ingredientsSodium bicarbonateActivated carbon
The invention discloses a prescription of piperacillin sodium and tazobactam sodium for injection, which comprises the following raw materials: 1000 parts of piperacillin sodium, 250 parts of tazobactam sodium, 25 parts of activated carbon, an appropriate amount of sodium hydrogen carbonate, and an appropriate amount of water for injection up to 6000 ml. The invention further discloses a technology of the piperacillin sodium and tazobactam sodium for injection, which comprises the following steps: selecting stainless steel utensils and performing dry heat sterilization treatment for later useafter washing clean; calculating the amount of the raw materials charged according to the ratio in the prescription, and preparing 1000 pieces of the piperacillin sodium and tazobactam sodium for injection, which comprise 1000 g of piperacillin sodium, 250 g of tazobactam sodium and 25 g of activated carbon respectively, according to the specification of 1.25g / piece; and preparing 1000 pieces of the piperacillin sodium and tazobactam sodium for injection, which comprise 2000 g of piperacillin sodium, 500 g of tazobactam sodium and 50 g of activated carbon respectively, according to the specification of 2.5g / piece. The prescription disclosed by the invention and the preparation technology using the prescription are simple in steps, and a higher quality piperacillin sodium tazobactam and sodium product for injection can be produced.
Owner:JIANGSU HAIHONG PHARMA
Compound pharmaceutical composition containing piperacillin sodium and tazobactam sodium and preparation method of compound pharmaceutical composition
ActiveCN105456268AGuaranteed stabilityImprove stabilityAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumDissolution
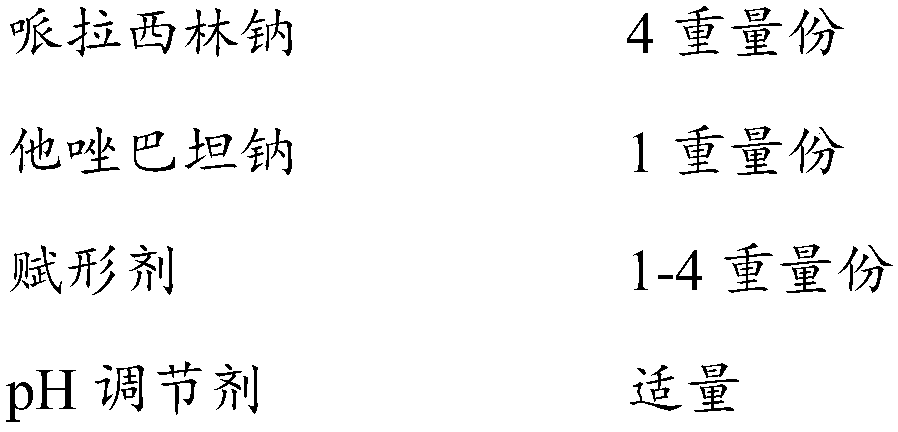
The invention discloses a compound pharmaceutical composition containing piperacillin sodium and tazobactam sodium. The compound pharmaceutical composition contains piperacillin sodium, tazobactam sodium, an excipient and a pH regulator, wherein the ratio of piperacillin sodium to tazobactam sodium is 4:1. Moreover, the invention also discloses a preparation method of the pharmaceutical composition. Compared with the prior art, the pharmaceutical composition disclosed by the invention not only is good in stability, low in impurity content and high in safety, but also is improved in dissolution rate and is more suitable for industrialization.
Owner:FUAN PHARM (GRP) CO LTD +1
Method for controlling acidity of piperacillin sodium and tazobactam sodium for injection
InactiveCN104083766AChange complianceImprove stabilityAntibacterial agentsInorganic non-active ingredientsPiperacillin Sodium/ Tazobactam SodiumFreeze-drying
The invention relates to a method for controlling the acidity of piperacillin sodium and tazobactam sodium for injection. The method is characterized by comprising the following steps of: (1) determining the weight ratio of piperacillin sodium to tazobactam sodium at 2:1; (2) determining the weight ratio of ion exchange resin to water at 1:25, mixing with one or two of piperacillin sodium and tazobactam sodium, and adding alkali in a salifying process while stirring, wherein the alkali is prepared into an alkali solution with certain concentration; (3) controlling the acidity of liquid medicine subjected to filter pressing at 5.8-6.2, filtering, and preparing filtrate into a liquid injection, an infusion solution, a powder injection and a freeze-dried powder injection according to a pharmaceutic conventional requirement. According to the method, the pH value is 6.13, the moisture is 0.7%, and the bacterial endotoxin content of every 1 mg of a product is less than 0.060 EU; the acidity of a finished product is 5.8-6.5, so that the stability of the product is enhanced, and the compliance of the medication of a patient is changed.
Owner:JIANGSU HAIHONG PHARMA
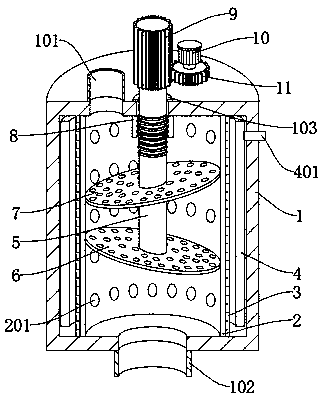
Sterile filtration system for piperacillin sodium and tazobactam sodium for injection
ActiveCN108721987APrevent leakageGuarantee sufficiencyHeatMoving filtering element filtersPiperacillin Sodium/ Tazobactam SodiumSterile environment
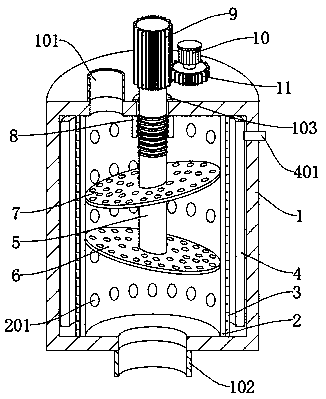
The invention discloses a sterile filtration system for piperacillin sodium and tazobactam sodium for injection. The sterile filtration system comprises a filter tube, wherein an inner tube is fixedlydisposed inside the filter tube; a sterilization hole is formed in the side wall of the inner tube in a penetration manner; the outer side of the inner tube is fixedly covered with a rubber ring; a steam spraying nozzle is disposed in the rubber ring in a penetration manner; and one end of the steam spraying nozzle is movably located in the sterilization hole. According to the invention, when a filtration process of production is needed to be ensured in a sterile environment, a large amount of steam is injected into a steam sleeve from a steam generator, and the steam is injected into the inner tube from the front end of the steam spraying nozzle, so that the sterilization treatment is performed; a liquid to be filtered is injected into the inner tube from a water inlet during filtration,then a motor is driven, and a gear is driven by the motor to rotate, so that a threaded shaft is driven to rotate, and under the action of a threaded sleeve, the threaded shaft can be enabled to moveup and down while rotating, thereby changing a pre-filter plate and a sterile filter plate, and ensuring the sufficiency of the filtration of the liquid; and the filtered liquid is discharged from awater outlet.
Owner:JIANGSU HAIHONG PHARMA
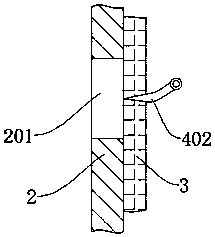
A kind of sterile filtration system for piperacillin sodium tazobactam sodium for injection
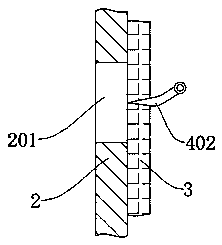
ActiveCN108721987BPrevent leakageGuarantee sufficiencyHeatMoving filtering element filtersPiperacillin Sodium/ Tazobactam SodiumRubber ring
Owner:JIANGSU HAIHONG PHARMA
Piperacillin sodium, tazobactam sodium and probenecid sodium three-component freeze-dried preparation for injection
PendingCN112076162AThere will be no difference in loadingPowder deliveryPeptide/protein ingredientsPiperacillin Sodium/ Tazobactam SodiumPhysical chemistry
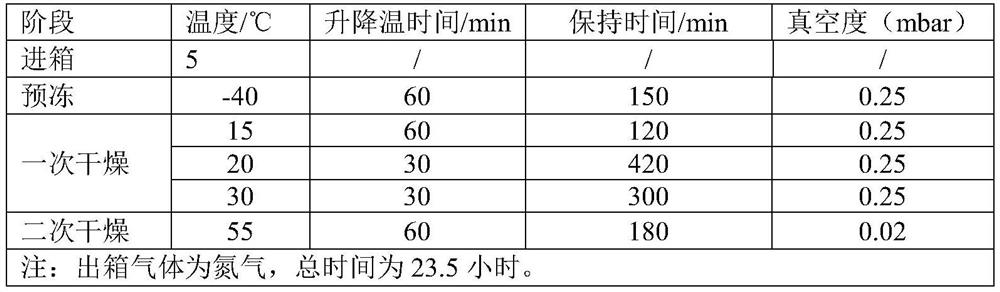
The invention relates to a piperacillin sodium, tazobactam sodium and probenecid sodium tripartite freeze-dried preparation for injection, which is characterized by comprising the following steps: adding piperacillin sodium, tazobactam sodium and probenecid sodium in a ratio of 8: (1-0.5): (1-2) raw material into water for injection for complete dissolution, regulating the pH value to 6.5-7.5, adding the water for injection to full dose, sterilizing, filtering and freeze-drying; wherein the plate layer of the freeze dryer is cooled to-40 DEG C from the room temperature at the speed of about 2DEG C / min, and heat preservation is conducted for 60-90 min; after sublimation drying, performing desorption drying, fully volatilizing moisture, performing judgment according to the pressure change condition in the freeze-drying box body, taking out the freeze-drying box body after being qualified, and the total freeze-drying time is 22 hours; and taking out the sample, capping and inspecting. The three components are completely mixed in the solution, the uniformity is high, the defects of the traditional sterile powder mixing process are overcome, the qualification rate of the finished product is greatly improved, and meanwhile, the activity of the three components is not damaged.
Owner:安徽康正康仁药业有限公司
Piperacillin sodium-tazobactam sodium medicine composition and preparation method thereof
ActiveCN103340866BLow polymer contentImprove stabilityAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumMass ratio
The invention belongs to the technical field of medicine, and in particular relates to a piperacillin sodium-tazobactam sodium medicine composition and a preparation method of the medicine composition. The medicine composition is a sterile powder injection; the mass ratio of the piperacillin sodium to the tazobactam sodium in the medicine composition is 4-20:1; the X-ray powder diffraction pattern of the piperacillin sodium measured by means of powder X-ray diffraction measurement method is shown in Figure 1; the chemical structural formula of the piperacillin sodium is shown in Formula (I); and the content of the piperacillin sodium polymer in the medicine composition provided by the invention is quite low and is not changed obviously in an accelerated test condition and in a long-term test condition. The medicine composition prepared from the piperacillin sodium and the tazobactam sodium provided by the invention also has the advantages of low polymer content and good stability.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Piperacillin sodium-tazobactam sodium medicinal composition microsphere injection
InactiveCN101890016BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryPiperacillin Sodium/ Tazobactam SodiumMicrosphere
The invention discloses piperacillin sodium-tazobactam sodium medicinal composition microsphere injection. The injection is characterized by consisting of piperacillin sodium, tazobactam sodium, polylactic acid-polyethylene glycol (PLA-PEG), PEG600, polysorbate 80 and mannitol and particularly consisting of 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4 to 9 parts of PLA-PEG,6 to 10 parts of PEG600, 1 to 3 parts of polysorbate 80 and 2 to 4 parts of mannitol. Compared with the prior art, the piperacillin sodium-tazobactam sodium medicinal composition microsphere injection prepared by the invention has the characteristics of good stability, high entrapment rate, high preparation technology repeatability, suitability for industrial production, uniform particle distribution, few solvent residue, good injectability and excellent sustained release property.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Piperacillin sodium-tazobactam sodium preparation for injection and preparation method thereof
ActiveCN104644637BImprove securityEasy to decompose and removeAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumTazobactam piperacillin
The invention discloses a pharmaceutical composition of piperacillin sodium and tazobactam sodium and a preparation method thereof. The pharmaceutical composition is a sterile powder injection, wherein the weight ratio of piperacillin sodium to tazobactam sodium is 2 ~4:1. The piperacillin sodium-tazobactam sodium sterile powder injection prepared by the invention has high stability and less impurities, and greatly improves the safety and effectiveness of clinical medication.
Owner:NORTH CHINA PHARMA COMPANY +2
Preparation method of piperacillin sodium and tazobactam sodium sterile powder injection
ActiveCN113209030AAvoid decompositionImprove stabilityAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumEngineering
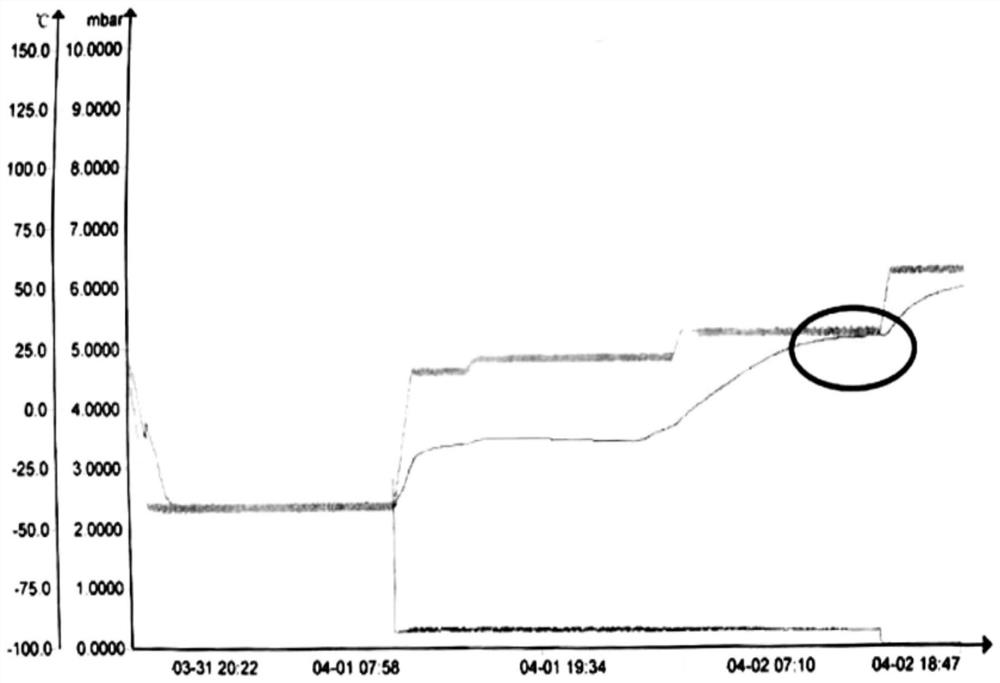
The invention provides a preparation method of a piperacillin sodium and tazobactam sodium sterile powder injection, belongs to the technical field of medicine preparation. The preparation method comprises the following steps of under the protection of an inert gas, taking water for injection, adding piperacillin sodium and tazobactam sodium for dissolution, performing decoloration, sterilization and filtration, and enabling the obtained feed liquid to be subjected to pre-freezing, four times of sublimation drying and desorption drying to obtain the piperacillin sodium and tazobactam sodium sterile powder injection. According to the invention, through slow heating and segmented drying, the decomposition of piperacillin sodium and tazobactam sodium is effectively inhibited, and the stability of the finished product is improved. The preparation method provided by the invention can effectively improve the drug stability, appearance shape and looseness of the finished product.
Owner:HAINAN GENERAL & KANGLI PHARMA
Method for preparing piperacillin sodium and tazobactam sodium freeze-dried powder by microreactor
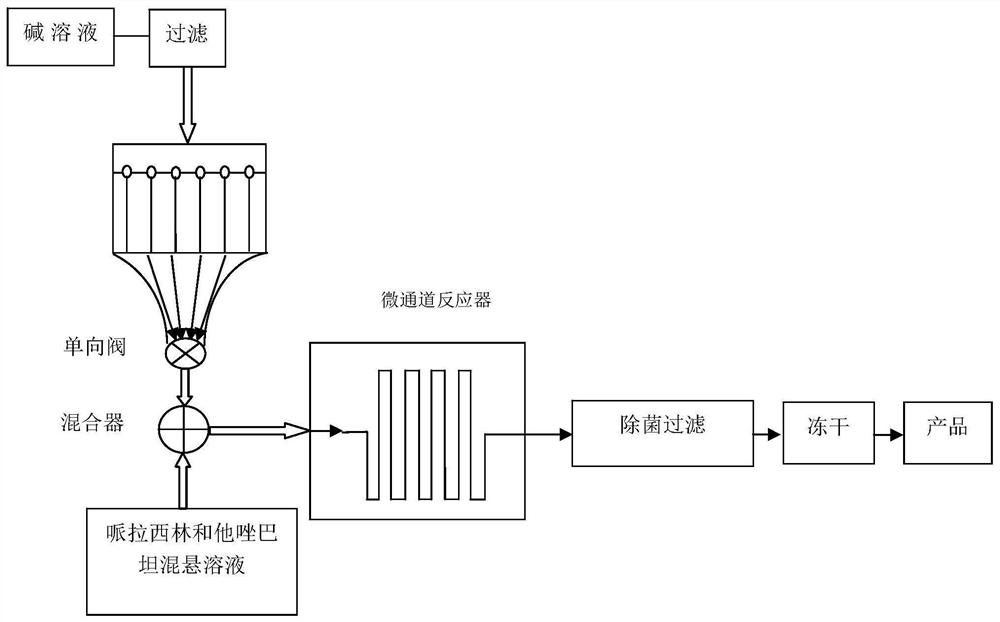
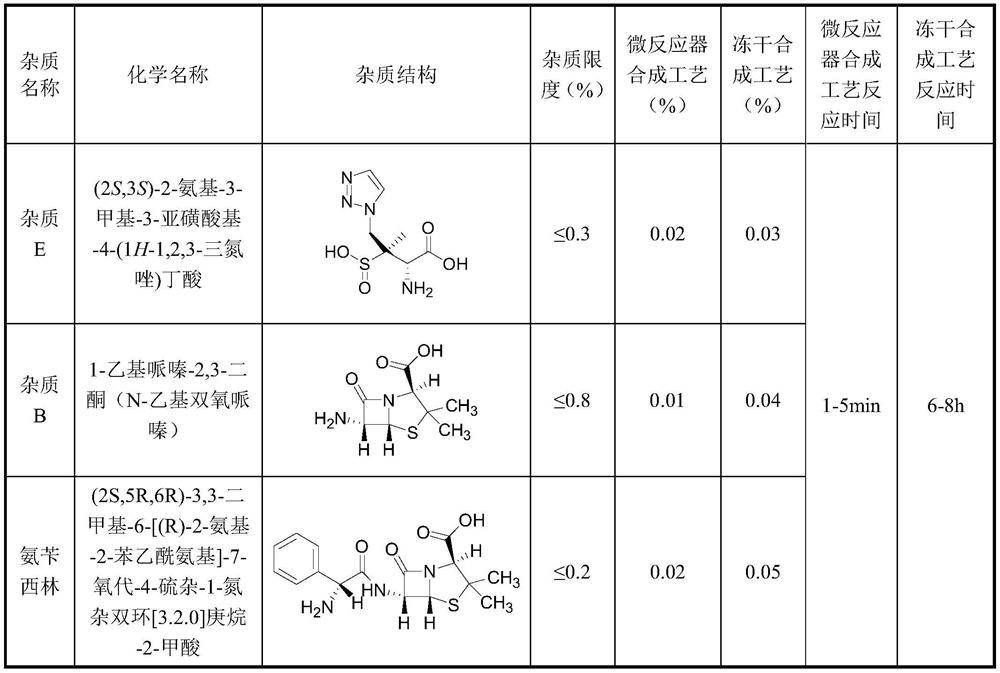
InactiveCN112057426AShort stayRapid responseAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumMicroreactor
The invention discloses a method for preparing piperacillin sodium and tazobactam sodium freeze-dried powder by a microreactor. According to the method, an inorganic alkali solution and a piperacillinand tazobactam suspension solution enter a mixer to be mixed together by controlling a certain flow rate and enter a microreactor to react, the mixture is taken out of the microreactor after the reaction is completed, and piperacillin sodium and tazobactam sodium freeze-dried powder is obtained after filtration, sterilization and freeze-drying. According to the method, rapid reaction of the microreactor is utilized, so that degradation impurity increase caused by too high local concentration of alkali liquor is avoided, nitrogen does not need to be introduced in the reaction process, carbon dioxide generated in the reaction process does not need to be removed by vacuumizing and is directly discharged into the air; and due to short residence time in a micro-reaction unit and rapid reaction, the spraying phenomenon caused by carbon dioxide is avoided, so that the reaction steps are greatly simplified, the production period is short, the degradation impurities are few, the product stability is good, and the production cost is low.
Owner:山东安信制药有限公司 +1
Removing method of polymer impurities in piperacillin sodium and tazobactam sodium for injection
InactiveCN111514101AEffective controlEnsure safetyAntibacterial agentsPharmaceutical delivery mechanismPiperacillin Sodium/ Tazobactam SodiumPhysical chemistry
The invention discloses a removing method of polymer impurities in piperacillin sodium and tazobactam sodium for injection. The removing method comprises the steps of adding a piperacillin sodium andtazobactam sodium freeze-drying agent for injection to a solvent for mandatory degradation to obtain a piperacillin sodium and tazobactam sodium mandatory degradation solution, performing placing fordefinite time, adding the piperacillin sodium and tazobactam sodium mandatory degradation solution after placing for definite time in an efficient gel chromatographic column, charging a mobile phase,and performing gradient elution. Through controlling the concentration of an eluent, column temperature, elution time and the proportion of the mobile phase, the polymer impurities in piperacillin sodium and tazobactam sodium for injection are removed, the polymer impurities in piperacillin sodium and tazobactam sodium are effectively controlled, and the safety of the piperacillin sodium and tazobactam sodium for injection is guaranteed.
Owner:JIANGSU HAIHONG PHARMA
Compound pharmaceutical composition of piperacillin sodium and tazobactam sodium and preparation method thereof
ActiveCN105456268BGuaranteed stabilityImprove stabilityAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumDissolution
The invention discloses a compound pharmaceutical composition containing piperacillin sodium and tazobactam sodium. The compound pharmaceutical composition contains piperacillin sodium, tazobactam sodium, an excipient and a pH regulator, wherein the ratio of piperacillin sodium to tazobactam sodium is 4:1. Moreover, the invention also discloses a preparation method of the pharmaceutical composition. Compared with the prior art, the pharmaceutical composition disclosed by the invention not only is good in stability, low in impurity content and high in safety, but also is improved in dissolution rate and is more suitable for industrialization.
Owner:FUAN PHARM (GRP) CO LTD +1
Preparation method of piperacillin sodium and tazobactam sodium freeze-drying agent for injection
PendingCN113750057AExtended reaction timeShort reaction timeAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumDrug product
The invention provides a preparation method of a piperacillin sodium and tazobactam sodium freeze-drying agent for injection. The preparation method comprises the following steps: preparing a citric acid solution and a buffer solution, adding tazobactam acid to form a sodium salt, and adding piperacillin acid to prepare piperacillin sodium to obtain a composite salt solution;adding sodium bicarbonate powder into a composite salt solution for multiple times in a stirring state to adjust a reaction state, and performing one-time constant volume treatment: cleaning a solution preparation container with 10%-20% ultrapure water and diluting the solution until raw materials and auxiliary materials are completely dissolved in a vacuumizing and stirring state; chelating the composite salt solution with EDTA-2Na to remove impurities so as to obtain a liquid medicine; and carrying out secondary constant volume: adjusting the pH value to 6.3-6.6, and then, carrying out low-temperature filling and freeze-drying, thereby obtaining the freeze-drying agent. Through an innovative raw material and auxiliary material adding method, stirring rate control and prolonging of raw material and auxiliary material reaction time, complete reaction of raw material medicines can be guaranteed, product content and insoluble particles can easily meet medicine quality requirements, and it is guaranteed that the insoluble particles of the product have no obvious growth trend over time.
Owner:江苏睿实生物科技有限公司
Pharmaceutical composition of piperacillin sodium-tazobactam sodium compound
InactiveCN107625772AImprove stabilizerGuaranteed curative effectAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumTAZOBACTAM SODIUM
The invention relates to a pharmaceutical composition of a piperacillin sodium-tazobactam sodium compound. By adopting sodium caprylate as a stabilizer, polysorbate as an emulsifier and trehalose as afrozen-dried supporting agent, the stability of piperacillin sodium and tazobactam sodium during preparation and storage can be effectively enhanced, and the efficacy and safety of clinical use can be guaranteed.
Owner:SUZHOU ERYE PHARMA CO LTD
Special piperacillin sodium-tazobactam sodium ultra-fine powder preparation and preparation method thereof
InactiveCN104856997AHigh puritySmall particlesAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumAlcohol
The present invention discloses a special piperacillin sodium-tazobactam sodium ultra-fine powder preparation and a preparation method thereof. The preparation method comprises: respectively dissolving a piperacillin sodium crude product and a tazobactam sodium crude product in water, respectively carrying out gel column chromatography, and respectively collecting the elution fractions of piperacillin sodium and tazobactam sodium by using an alkyl alcohol aqueous solution as an eluant; carrying out chromatography on the obtained elution fractions through a reverse phase chromatography column, and respectively collecting the elution fractions of piperacillin sodium and tazobactam sodium; respectively drying 99% of the elution fractions of piperacillin sodium and tazobactam sodium by using a vacuum drying method to prepare the raw materials such as the high purity piperacillin sodium and the high purity tazobactam sodium; mixing the obtained high purity piperacillin sodium and the obtained high purity tazobactam sodium according to a weight ratio to obtain the high purity piperacillin sodium-tazobactam sodium mixing powder raw material; and carrying out jet crushing on the obtained piperacillin sodium-tazobactam sodium mixing powder raw material to obtain the ultra-fine powder, and carrying out freeze drying.
Owner:杭州长典老一元健康管理有限公司 +1
A kind of preparation method of piperacillin sodium tazobactam sodium for injection
ActiveCN105616415BAvoid decompositionImprove acidity stabilityAntibacterial agentsPowder deliverySodium bicarbonatePiperacillin Sodium/ Tazobactam Sodium
The invention discloses a preparation method of piperacillin sodium and tazobactam sodium for injection. The preparation method comprises the following steps: adding water into a blending tank, cooling to 5-10 DEG C, adding citric acid, then slowly adding sodium bicarbonate, and after reacting, reading the pH value and adjusting the pH value to be 6.3-6.6; adding piperacillin acid into the blending tank, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, adding tazobactam acid, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, carrying out vacuum removal on carbon dioxide gas by suction, and after the feed liquid in the blending tank is stabilized, reading the pH value and adjusting the pH value to be 6.0-6.5; and carrying out aseptic filtration and freeze-drying to obtain raw powder of piperacillin sodium and tazobactam sodium for injection. According to the preparation method, by adding salified citric acid, the acidity stability of the product is increased, the decomposition of piperacillin sodium and tazobactam sodium is inhibited, and the storage stability of the product is increased.
Owner:山东安信制药有限公司
Piperacillin sodium and tazobactam sodium co-amorphous substance and preparation method thereof
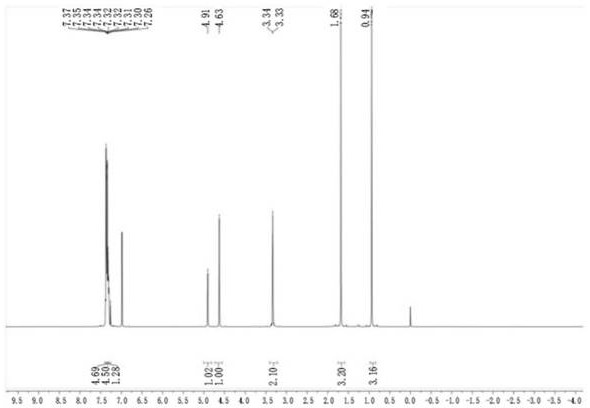
ActiveCN112409381AImprove stabilityHigh purityOrganic chemistryPiperacillin Sodium/ Tazobactam SodiumOrganosolv
The invention discloses a piperacillin sodium and tazobactam sodium co-amorphous substance and a preparation method thereof. The preparation method comprises the steps of dissolving piperacillin and tazobactam into an organic solvent in proportion, dropwise adding dissolved organic sodium salt (or inorganic sodium salt) in an anhydrous state, and separating out piperacillin sodium and tazobactam sodium mixed powder after reaction. The mixed powder is determined as a hydrogen bond bonded co-amorphous substance of piperacillin sodium and tazobactam sodium through powder X diffraction, infrared spectroscopy and DSC analysis. Compared with the traditional generation mode, the piperacillin sodium and tazobactam sodium co-amorphous substance prepared by the method has the advantages of low impurity content and good stability, the medication safety can be better improved, and the generation of adverse reactions is reduced.
Owner:山东安信制药有限公司 +1
Method for reducing visible foreign matters in piperacillin sodium and tazobactam sodium for injection
InactiveCN104095852AImprove securityAntibacterial agentsPharmaceutical delivery mechanismForeign matterFreeze-drying
A method for reducing visible foreign matters in piperacillin sodium and tazobactam sodium for injection is characterized by comprising the steps as follows: 1), bottle washing equipment is cleaned; 2), piperacillin sodium and tazobactam sodium are taken at a weight ratio of 8:1; and 3), ion exchange resin and water are taken at a weight ratio of 1: 35 and jointly or independently mixed with piperacillin sodium and tazobactam sodium for stirring, filtering and filter pressing, and filter liquor is prepared into an aqueous injection, an infusion solution, a powder injection or a freeze-dried powder injection according to conventional requirements of pharmaceutics. According to the invention, the number of visible foreign matters in each bottle is reduced to be less than 4 from less than 10 and exceeds the specified requirements of the drug standard of Chinese pharmacopoeia, and the safety of drug application is greatly improved.
Owner:JIANGSU HAIHONG PHARMA
Method for improving yield of injection piperacillin sodium and tazobactam sodium
InactiveCN104083371AImprove stabilityQuality assuranceAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumDrug product
The invention provides a method for improving the yield of injection piperacillin sodium and tazobactam sodium. The method is characterized by comprising the following steps: (1) weighing piperacillin sodium and tazobactam sodium in a weight ratio of 2:1; (2) weighing ionic exchange resin and water in a weight ratio of 1:30, jointly or independently mixing with piperacillin sodium and tazobactam sodium, stirring at a speed of 1300-1500 revolutions / minute by adopting a double-section paddle type at an angle of 15 degrees formed by leading a paddle to be vertical to the liquid level, filtering by use of a 120-mesh filter screen, and preparing injections, infusion solutions, powder injections or lyophilized powder. According to the method, the yield of the injection piperacillin sodium and tazobactam sodium is 90.0 percent, the pH value is 6.3, the moisture is less than 0.7 percent, the content of piperacillin in 1mg of injection piperacillin sodium and tazobactam sodium is 882 mu g, and the content of tazobactam in 1mg of injection piperacillin sodium and tazobactam sodium is 112 mu g, so that the drug stability is further improved, and the quality of medicines in validity can be guaranteed.
Owner:JIANGSU HAIHONG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com