Production method of piperacillin sodium tazobactam sodium freeze-drying preparation for injection
A technology of piperacillin sodium and tazobactam sodium, applied in the field of medicine, can solve the problems of poor uniformity, high production cost, slow reaction speed and the like, and achieve the effects of improving stability, good product quality and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
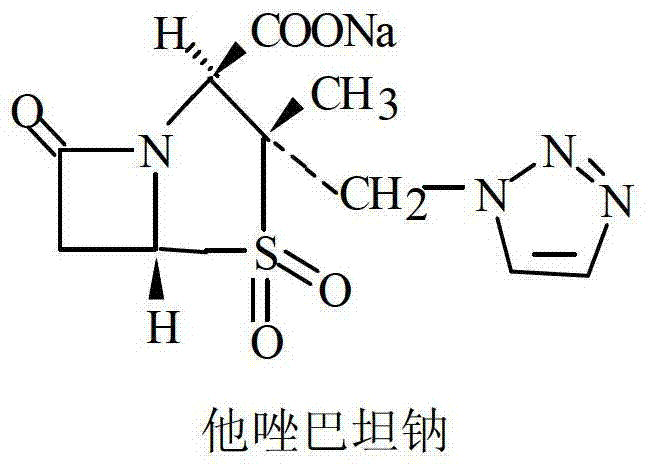
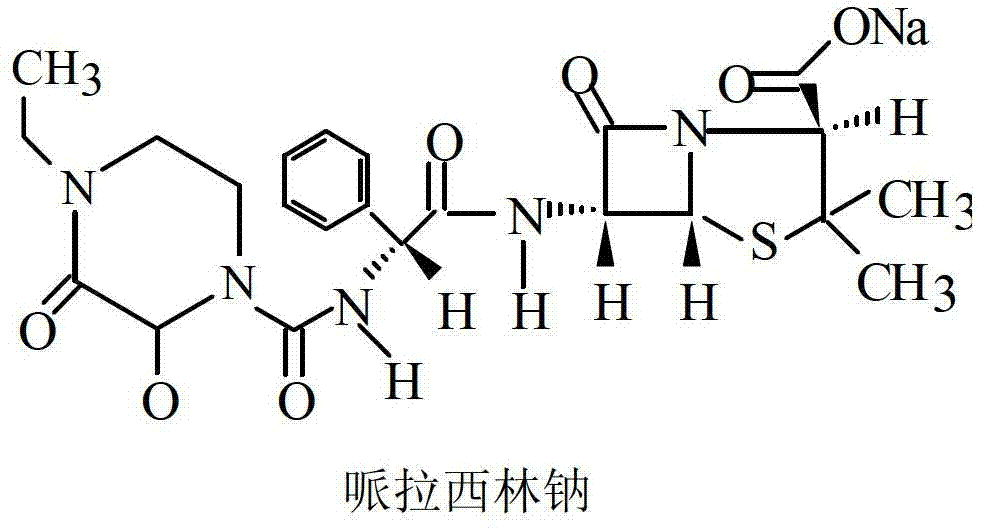
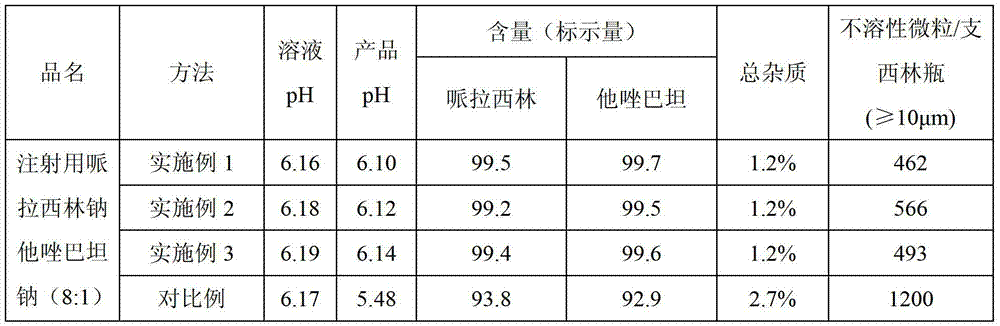
[0025] Add 150ml of water, 80g of piperacillin acid monohydrate (molecular weight: 535.54, equivalent to 14.94cMol), and 10g of tazobactamic acid (molecular weight: 300.28, equivalent to 3.33cMol) in a 1L reactor. Dissolve 7.308g of sodium hydroxide (equivalent to 18.27cMol) in 350ml of water and slowly add to the reaction kettle with nitrogen at the bottom of the kettle and good stirring. During the alkali feeding process, the temperature of the reaction solution is controlled at ≤ 5°C, and the temperature of the feed liquid is controlled between 5-10°C after the feeding is completed. Finally adjust the pH to between 5.0-7.0, set the shelf temperature to -30°C, drop the shelf temperature to -40°C within 120 minutes, set the prefreeze temperature below -40°C, and stabilize 4- 8 hours. Vacuum to ≤-0.08MPa, heat up the plate layer to 55°C, and sublimation drying time is 6-12 hours. The vacuum degree of the second-stage drying of the product reaches ≤-0.05MPa, the temperature i...
Embodiment 2
[0027] Add 200ml of water, 160g of piperacillin acid monohydrate (equivalent to 29.88cMol), 20g of tazobactamic acid (equivalent to 6.66cMol) into a 2L reaction kettle, and pour nitrogen gas at the bottom of the reaction kettle under good stirring. Dissolve 14.62g of sodium hydroxide in 400ml of water (equivalent to 36.54cMol) and slowly add it to the reactor. During the alkali feeding process, the temperature of the reaction solution is controlled at ≤ 5°C, and the temperature of the feed liquid is controlled between 5-10°C after the feeding is completed. Finally adjust the pH to between 5.0-7.0, set the shelf temperature to -30°C, drop the shelf temperature to -40°C within 120 minutes, set the prefreeze temperature below -40°C, and stabilize 4- 8 hours; evacuate to ≤-0.08MPa, and the temperature of the plate layer rises from below -45°C to 55°C. The sublimation drying time is 6-12 hours, the vacuum degree of the second-stage drying of the product reaches ≤-0.05MPa, the temp...
Embodiment 3
[0029]Add 300ml of water, 200g of piperacillin acid monohydrate (equivalent to 37.35cMol), 25g of tazobactamic acid (equivalent to 8.33cMol) into a 2L reaction kettle, and pour nitrogen at the bottom of the reaction kettle under good stirring. Dissolve 18.272g of sodium hydroxide in 350ml of water (equivalent to 45.68cMol) and slowly add it to the reactor. During the alkali feeding process, the temperature of the reaction solution is controlled at ≤ 5°C, and the temperature of the feed liquid is controlled between 5-10°C after the feeding is completed. Finally adjust the pH to between 5.0-7.0, set the shelf temperature to -30°C, drop the shelf temperature to -40°C within 120 minutes, set the prefreeze temperature below -40°C, and stabilize 4- 8 hours; evacuate to ≤-0.08MPa, and the temperature of the plate layer rises from below -45°C to 55°C. The sublimation drying time is 6-12 hours, the vacuum degree of the second-stage drying of the product reaches ≤-0.05MPa, the temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com