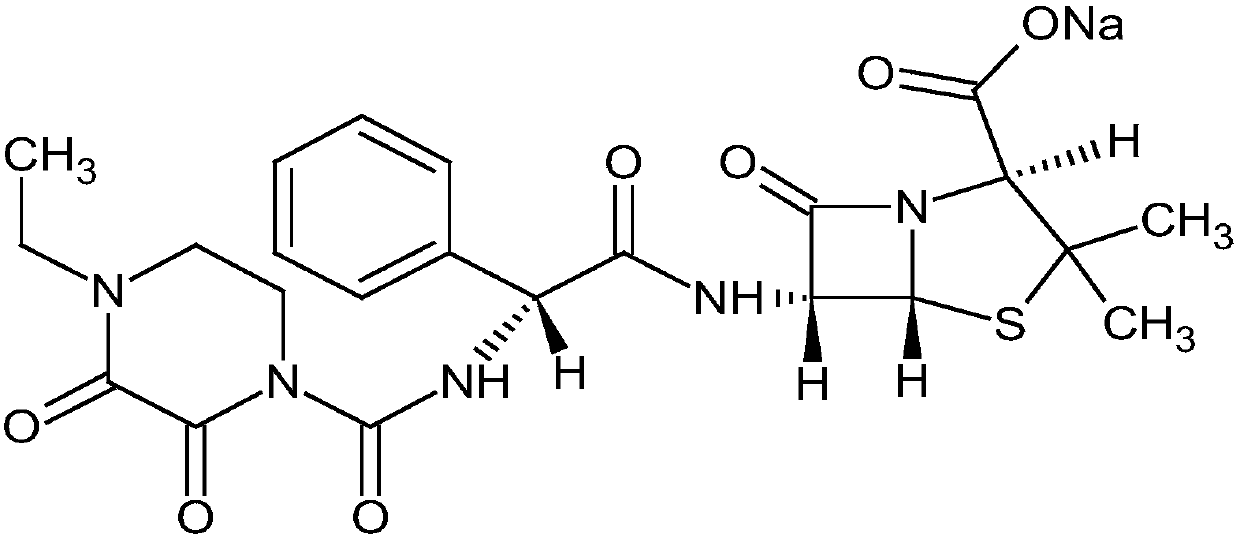
Pharmaceutical composition of piperacillin sodium-tazobactam sodium compound
A technology of tazobactam sodium and piperacillin sodium, which is applied in the direction of active ingredients of heterocyclic compounds, antibacterial drugs, freeze-dried transportation, etc., can solve the problems of poor stability of piperacillin sodium and tazobactam sodium, and achieve Guaranteed efficacy and safety, improved stability, and rapid dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of Piperacillin Sodium-Tazobactam Sodium (4:1) Suspension Powder Injection
[0020] Prescription (100 bottles):
[0021] Piperacillin Sodium 50g
[0022] Tazobactam Sodium 12.5g
[0023] Polysorbate 50g
[0024] Sodium Caprylate 50g
[0025] Trehalose 100g
[0026] Preparation
[0027] Under the protection of an inert gas, polysorbate and sodium octanoate are dissolved in water for injection, then piperacillin sodium and tazobactam sodium are added, mixed evenly, heated and stirred in a water bath at 90°C until molten. The above liquid was kept warm at 70-90°C and sheared and stirred for 10 minutes with a tissue masher at a speed of 15000r / min to obtain a primary emulsion, which was then circulated and emulsified by a high-pressure homogenizer for 4 times to obtain an emulsion. Trehalose is added to the emulsion, dissolved, filtered, subpackaged, and freeze-dried to obtain piperacillin sodium-tazobactam sodium suspension powder for injectio...
Embodiment 2
[0028] Example 2 Preparation of Piperacillin Sodium-Tazobactam Sodium (4:1) Suspension Powder Injection
[0029] Prescription (200 bottles):
[0030] Piperacillin Sodium 100g
[0031] Tazobactam Sodium 25g
[0032] Polysorbate 100g
[0033] Sodium Caprylate 100g
[0034] Trehalose 200g
[0035] Preparation
[0036] Under the protection of an inert gas, polysorbate and sodium octanoate are dissolved in water for injection, then piperacillin sodium and tazobactam sodium are added, mixed evenly, heated and stirred in a water bath at 90°C until molten. The above liquid was kept warm at 70-90°C and sheared and stirred for 10 minutes with a tissue masher at a speed of 15000r / min to obtain a primary emulsion, which was then circulated and emulsified by a high-pressure homogenizer for 4 times to obtain an emulsion. Trehalose is added to the emulsion, dissolved, filtered, subpackaged, and freeze-dried to obtain piperacillin sodium-tazobactam sodium suspension powder for inject...
Embodiment 3
[0037] Embodiment 3 long-term stability test
[0038] Batch number 1: 160601 Batch size: 2000 bottles Specification: 1.125g / bottle Packaging: vials
[0039] Inspection conditions: 25±2℃ / 60±10%RH
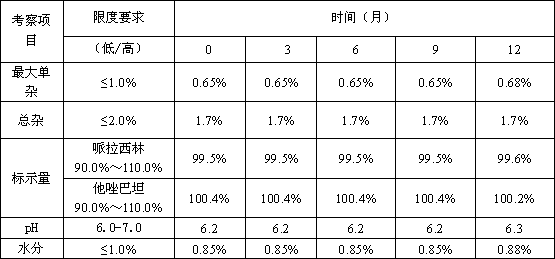
[0040]
[0041] Through the long-term stability test, it can be found that the composition of piperacillin sodium and tazobactam sodium of the present invention has good stability during preparation and storage, which ensures the curative effect and safety of clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com