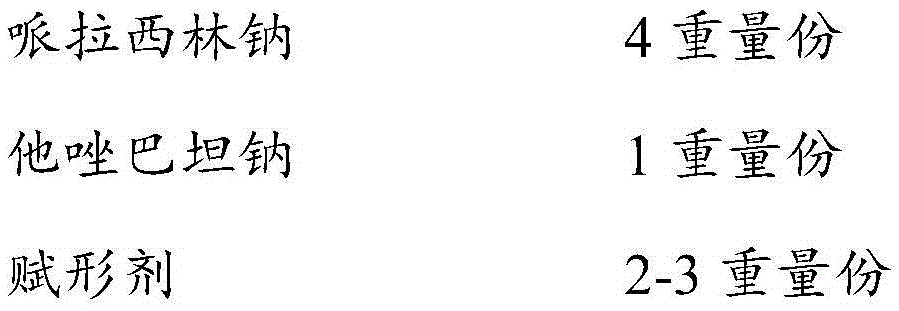Compound pharmaceutical composition containing piperacillin sodium and tazobactam sodium and preparation method of compound pharmaceutical composition
A technology of tazobactam sodium and piperacillin sodium, which is applied in the directions of drug delivery, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., can solve the problem of large number of impurities, high substance content, poor stability, etc. The problem is to overcome the complex formula, simple preparation process and small fluctuation range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prescription Screening of Piperacillin Sodium and Tazobactam Sodium
[0037] Taking the dissolution time as an index and using water for injection as a solvent, the preliminary selection of the prescription was carried out. The results of the investigation are shown in Table 1:
[0038]
[0039]
[0040] The results showed that when the dissolution time was taken as an index, the pH value was adjusted with citric acid-sodium citrate buffer solution in the formula of piperacillin sodium+tazobactam sodium+mannitol+lactose (4:1:2:1), The dissolution time is the shortest.
Embodiment 2
[0042] Prescription 1.25g, 1000 bottles
[0043]
[0044] Dissolve the prescribed amount of piperacillin sodium, tazobactam sodium, mannitol, and lactose in 1600ml of water for injection. After the dissolution is complete, adjust the pH value to 6.0-6.4 with citric acid-sodium citrate, and add 400ml of Add water for injection, add activated carbon for needles to absorb the heat source, sterilize and filter with membrane filters with 0.45 μm and 0.22 μm pore size respectively, put the sterile solution into bottles, and freeze-dry it.
Embodiment 3
[0050] Prescription 2.5g, 1000 bottles
[0051]
[0052] Dissolve the prescribed amount of piperacillin sodium, tazobactam sodium, mannitol, and lactose in 3200ml of water for injection. After the dissolution is complete, adjust the pH value to 5.8-6.2 with citric acid-sodium citrate, and add 800ml of Add water for injection, add activated carbon for needles to absorb the heat source, sterilize and filter with membrane filters with 0.45 μm and 0.22 μm pore size respectively, put the sterile solution into bottles, and freeze-dry it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com