Piperacillin sodium, tazobactam sodium and probenecid sodium three-component freeze-dried preparation for injection
A technology for zobactam sodium probenecid sodium and piperacillin sodium, applied in the field of medicine, can solve the problems of poor fluidity, easy occurrence of unqualified, strong hygroscopicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
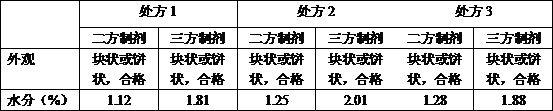
[0029] The prescription ratio of the tripartite preparation is: piperacillin sodium: tazobactam sodium: probenecid sodium is 8:1:1.
[0030] 1. Three-party preparation solution 1 (piperacillin sodium + probenecid sodium + tazobactam sodium) preparation:
[0031] The total amount of the solution is 2,500ml, which contains: piperacillin sodium 500 g (dry, pure), probenecid sodium 62.5 g (dry, pure), tazobactam sodium 62.5 g (dry, pure) Pure meter), the pH value of the solution is 6.0-7.5;
[0032] 2. Compound preparation solution 2 (piperacillin sodium) preparation:
[0033] The total amount of the solution is 2,500ml, which contains: 500 g of piperacillin sodium (dry and pure), 62.5 g of tazobactam sodium (dry and pure), and the pH value of the solution is 6.0-7.5. The difference between solution 1 and solution 2 is that the former contains 125 g of probenecid sodium and the latter does not.
[0034] 3. Use 10 ml vials, 500 vials each contain 5 ml of solution 1, which contai...
Embodiment 2
[0036] The prescription ratio of the tripartite preparation is: piperacillin sodium: tazobactam sodium: probenecid sodium is 8:0.5:2.
[0037] 1. Three-party preparation solution 1 (piperacillin sodium + probenecid sodium + tazobactam sodium) preparation:
[0038] The total amount of the solution is 2,500ml, which contains: piperacillin sodium 500 g (dry, pure), probenecid sodium 125 g (dry, pure), tazobactam sodium 31.25 g (dry, pure) Pure meter), the pH value of the solution is 6.0-7.5;
[0039] 2. Compound preparation solution 2 (piperacillin sodium) preparation:
[0040] The total amount of the solution is 2,500ml, which contains: 500 g of piperacillin sodium (dry and pure), 31.25 g of tazobactam sodium (dry and pure), and the pH value of the solution is 6.0-7.5. The difference between solution 1 and solution 2 is that the former contains 125 g of probenecid sodium and the latter does not.
[0041] 3. Use 10 ml vials, 500 vials each contain 5 ml of solution 1, which con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com