Piperacillin sodium and tazobactam sodium co-amorphous substance and preparation method thereof
A technology of tazobactam sodium and piperacillin sodium, which is applied in the field of medicine and chemical industry, can solve the problems of poor stability and no co-amorphous matter in the preparation process, and achieve the effect of good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
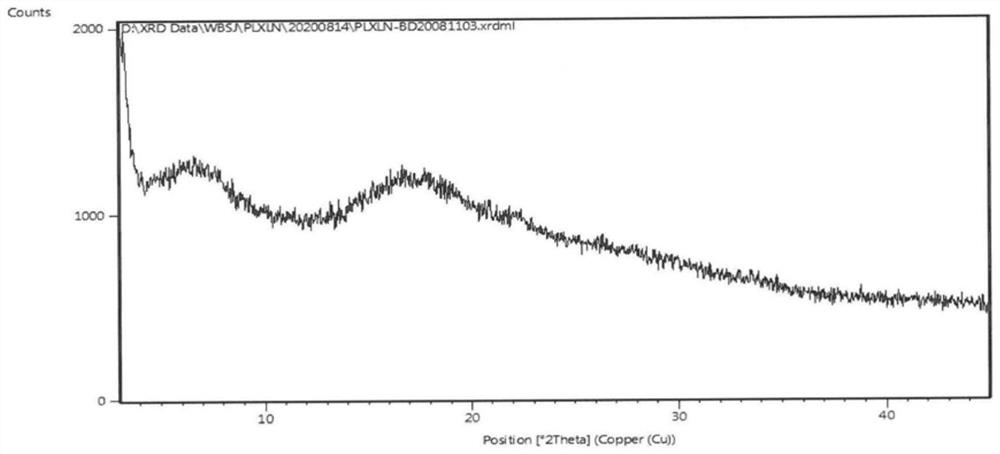
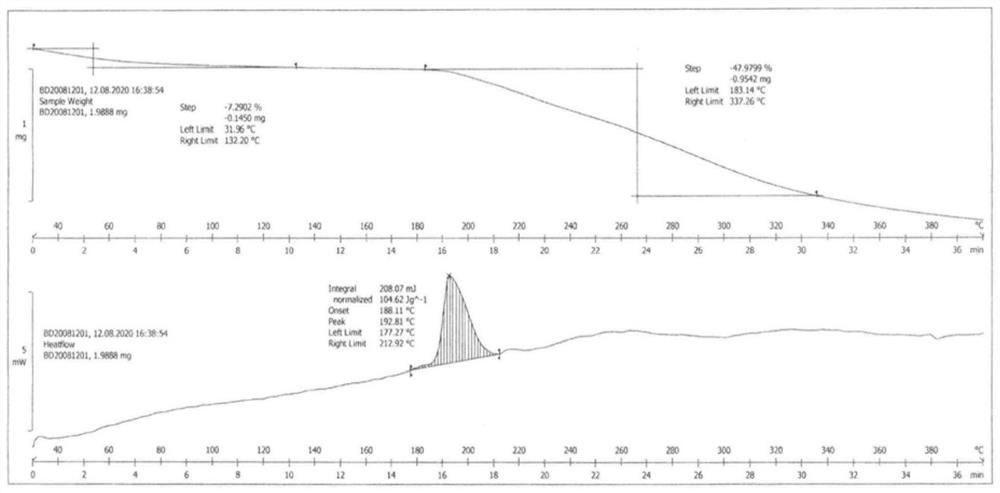
[0040]Weigh 10.0g of tazobactam and 80.0g of piperacillin, add 1200ml of acetone to dissolve, slowly add ethyl acetate solution of sodium isooctanoate (34.3g of sodium isooctanoate in 275ml of ethyl acetate) dropwise at 20-25°C, drop After the addition, stir for 2-2.5 hours, filter, and vacuum-dry at 40-50°C for 4-5 hours to obtain 85.2 g of piperacillin sodium and tazobactam sodium co-amorphous substance, yield 93.29%, HPLC: 99.77% . Residual solvent: acetone 320ppm, ethyl acetate 879ppm. The product is analyzed by powder X-ray diffraction, infrared, and DSC, and the results are as follows: figure 1 , 2 and 5.
[0041] Such as figure 1 As shown, using Cu-Ka radiation, there is no sharp diffraction peak in the X-ray powder diffraction (XRPD) figure represented by 2θ angle, indicating that it is not a crystalline state, but an amorphous state.
[0042] Such as figure 2 As shown, using an aluminum crucible, under flowing nitrogen, in a closed cup with pinholes, at a heati...
Embodiment 2
[0046] Weigh 10.0g tazobactam and 80.0g piperacillin, add 1000ml methyl ethyl ketone to dissolve, slowly add sodium formate butyl acetate solution (13.2g sodium formate dissolved in 105ml butyl acetate) dropwise at 20~25°C, after the dropwise addition is completed, Stir for 2-3 hours, filter, and vacuum-dry at 40-50°C for 4-5 hours to obtain 84.5 g of piperacillin sodium and tazobactam sodium co-amorphous substance, yield 92.52%, HPLC: 99.61%. Residual solvent: butanone 89ppm, butyl acetate 680ppm.
Embodiment 3
[0048] Weigh 10.0g tazobactam and 80.0g piperacillin, add 900ml cyclohexanone to dissolve, slowly add sodium acetate methyl acetate solution (18.2g sodium acetate dissolved in 150ml methyl acetate) dropwise at 25-30°C, drop After the addition, stir for 2-3 hours, filter, and vacuum-dry at 40-50°C for 4-6 hours to obtain 84.8 g of piperacillin sodium and tazobactam sodium co-amorphous substance, yield 92.85%, HPLC: 99.72% . Residual solvent: cyclohexanone 320ppm, methyl acetate 620ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com