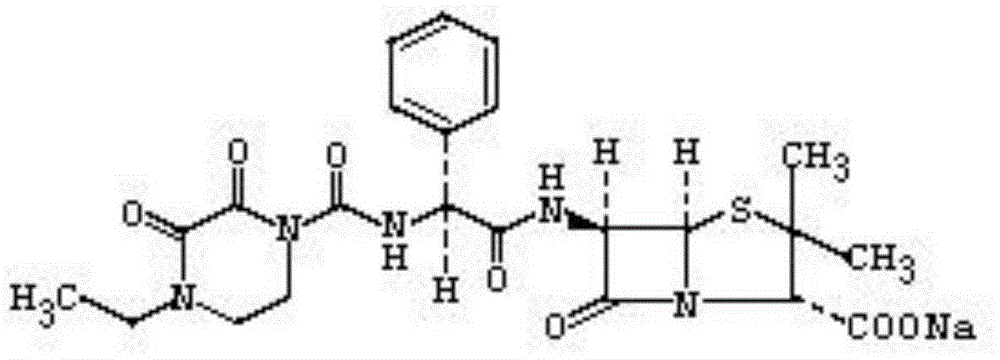
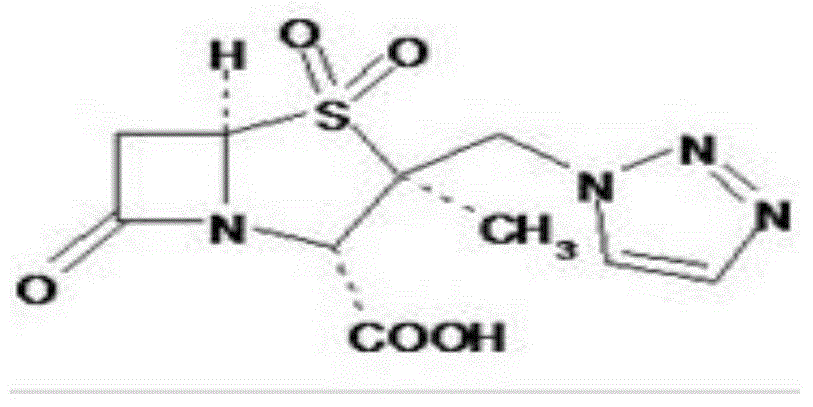
Special piperacillin sodium-tazobactam sodium ultra-fine powder preparation and preparation method thereof
A technology of tazobactam sodium and piperacillin sodium, applied in the field of medicine, can solve the problems of low purity of active ingredients, large side effects, etc., and achieve the effects of good chemical reactivity, less impurities, and reduced drug consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve the crude piperacillin sodium and tazobactam sodium in water respectively, respectively go through cross-linked sephadex column gel column chromatography, and use 75% ethanol solution as eluent to collect piperacillin The eluted fraction with 95% content of cillin sodium and tazobactam sodium; the obtained eluted fraction is subjected to reverse phase chromatography column chromatography with C4 as a filler, and 60% ethanol aqueous solution is used as an eluent to collect piperacillin respectively. Sodium and tazobactam sodium content of 99% elution fraction.
[0027] Then, 99% of the eluted components of the gained piperacillin sodium and tazobactam sodium were dried for 15 hours in a vacuum drying oven with a vacuum degree of 0.06 MPa at 50° C. to obtain high-purity piperacillin sodium and high-purity piperacillin sodium. Pure tazobactam sodium raw material, and mix high-purity piperacillin sodium and high-purity tazobactam sodium raw material in a ratio of 3...
Embodiment 2
[0031] Dissolve the crude piperacillin sodium and tazobactam sodium in water respectively, respectively go through cross-linked Sephadex column gel column chromatography, use 90% ethanol solution as eluent, collect piperacillin respectively The eluted fraction with 95% content of cillin sodium and tazobactam sodium; the obtained eluted fraction is subjected to reverse phase chromatography column chromatography with C4 as filler, and 75% ethanol aqueous solution is used as eluent to collect piperacillin respectively Sodium and tazobactam sodium content of 99% elution fraction.
[0032] Then, 99% of the eluted components of the gained piperacillin sodium and tazobactam sodium were dried for 15 hours in a vacuum drying oven with a vacuum degree of 0.06 MPa at 50° C. to obtain high-purity piperacillin sodium and high-purity piperacillin sodium. Pure tazobactam sodium raw material, and mix high-purity piperacillin sodium and high-purity tazobactam sodium raw material in a ratio of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com