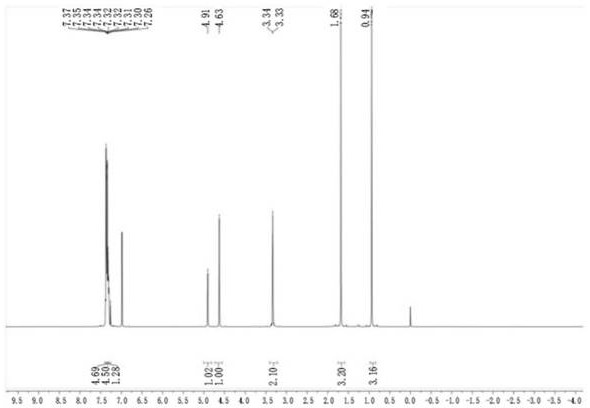
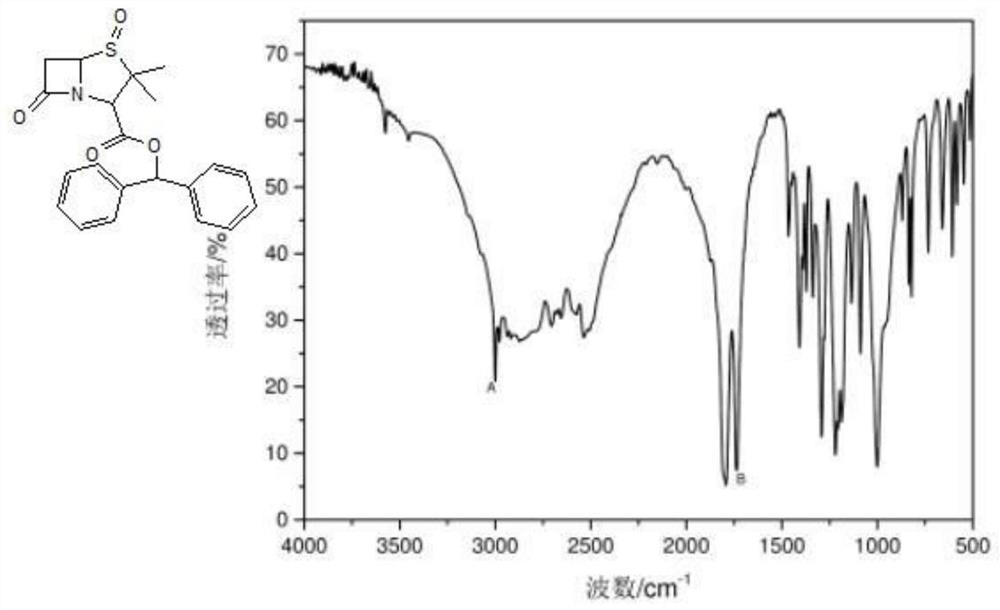
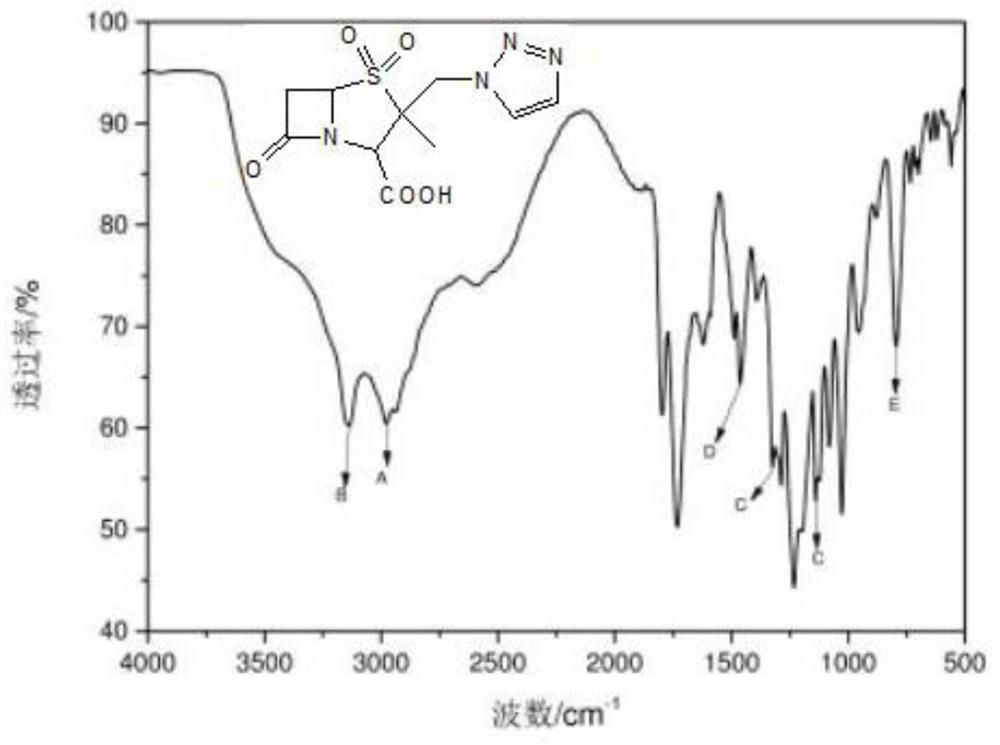
Preparation method of piperacillin sodium and tazobactam sodium sterile powder injection
A technology of tazobactam sodium and piperacillin sodium, which is applied in the direction of medical preparations containing active ingredients, antibacterial drugs, powder delivery, etc., and can solve the problem of poor stability and stable The stability needs to be improved and other issues, to achieve the effect of increasing the stability of the finished product, reducing the production cost and ensuring the singleness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1 A kind of synthetic method of tazobactam sodium
[0076] This embodiment is a synthetic method of tazobactam sodium, and the specific synthetic process includes the following steps carried out in sequence:
[0077] 1) Synthesis of diphenylmethyl penicillanic acid sulfoxide
[0078] After mixing 600mL of acetone and 100mL of water, add 108g (0.5mol) of 6-aminopenicillanic acid (6-APA), stir and cool down to -15°C, add 184g of saturated hydrochloric acid aqueous solution, and slowly add it dropwise while maintaining -15°C Hypophosphorous acid aqueous solution with a concentration of 50wt% [prepared by adding 62.7g (0.95mol) hypophosphorous acid to 62.7mL of ice water] was added dropwise for 1.5 hours. Sodium nitrite aqueous solution [prepared by adding 65.55g (0.95mol) sodium nitrite to 152mL ice water], add dropwise for 2 hours, after dropping, keep at -15°C for deamination reaction until the residual amount of 6-APA is less than 1% ( TLC detection), add 2...
Embodiment 2~5
[0109] The synthetic method of embodiment 2~5 tazobactam sodium
[0110] Examples 2 to 5 are respectively a synthetic method of tazobactam sodium, and their steps are basically the same as in Example 1, the difference is only in the amount of raw materials and process parameters, see Table 1 for details:
[0111] List of each technological parameter in the embodiment 2~5 of table 1
[0112]
[0113]
[0114]
Embodiment 6
[0115] Example 6 Preparation method of piperacillin sodium-tazobactam sodium sterile powder injection In this example, the tazobactam sodium M1 synthesized in Example 1 was used to prepare a piperacillin sodium-tazobactam sodium-free Bacteria powder injection, the specific preparation process includes the following steps carried out in sequence:
[0116] Take water for injection, boil it and let it cool for later use;
[0117] Under the protection of nitrogen, take 2000mL of boiled water for injection, add 100g of piperacillin sodium and 12.5g of tazobactam sodium, stir to dissolve, add 1g of activated carbon for needles, stir for 30min to decolorize, filter to remove carbon, and then use 0.22μm micro The pore filter membrane is sterilized and filtered to obtain the medicinal solution for subsequent use;
[0118] Take the liquid medicine and pack it according to the required specifications (in this embodiment, the powder injection specification is 2.25g / bottle), half-stoppere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com