Liposome injection of pharmaceutical composition comprising piperacillin sodium and tazobactam sodium
A technology of tazobactam sodium and piperacillin sodium, which is applied in the field of medicine, can solve the problems of drug quality degradation, poor solution stability, and easy deterioration of active ingredients, and achieve good stability, simple preparation process, and convenient clinical use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
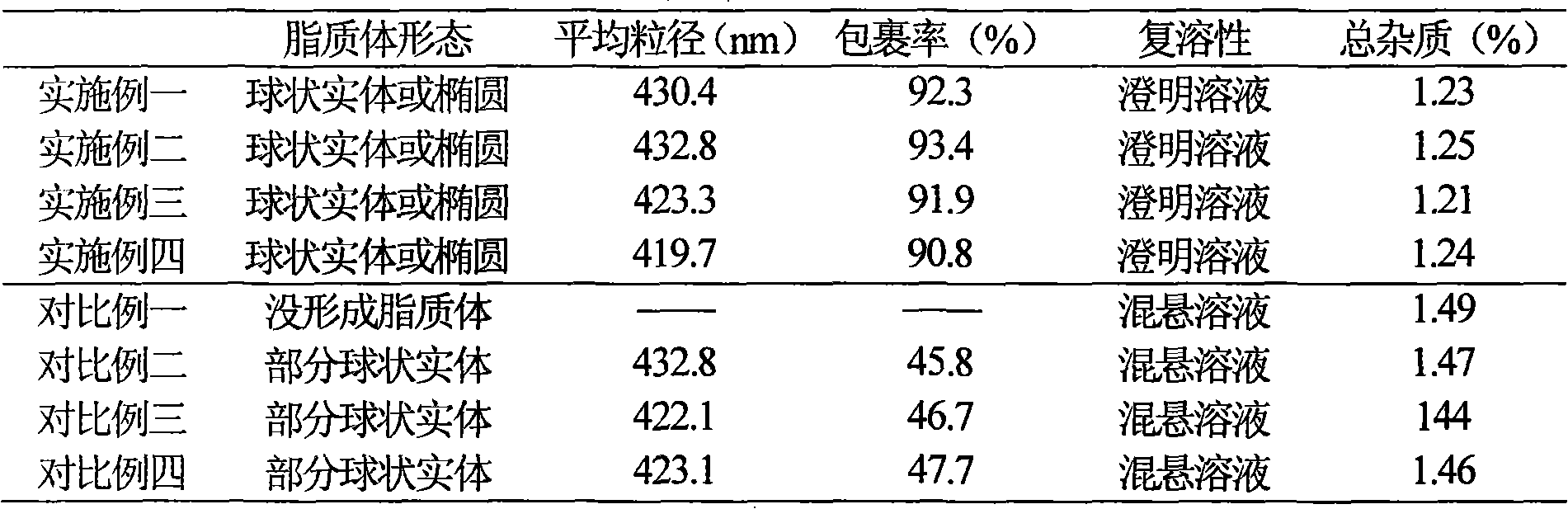
[0031] Example 1 Preparation of piperacillin sodium tazobactam sodium pharmaceutical composition liposome injection
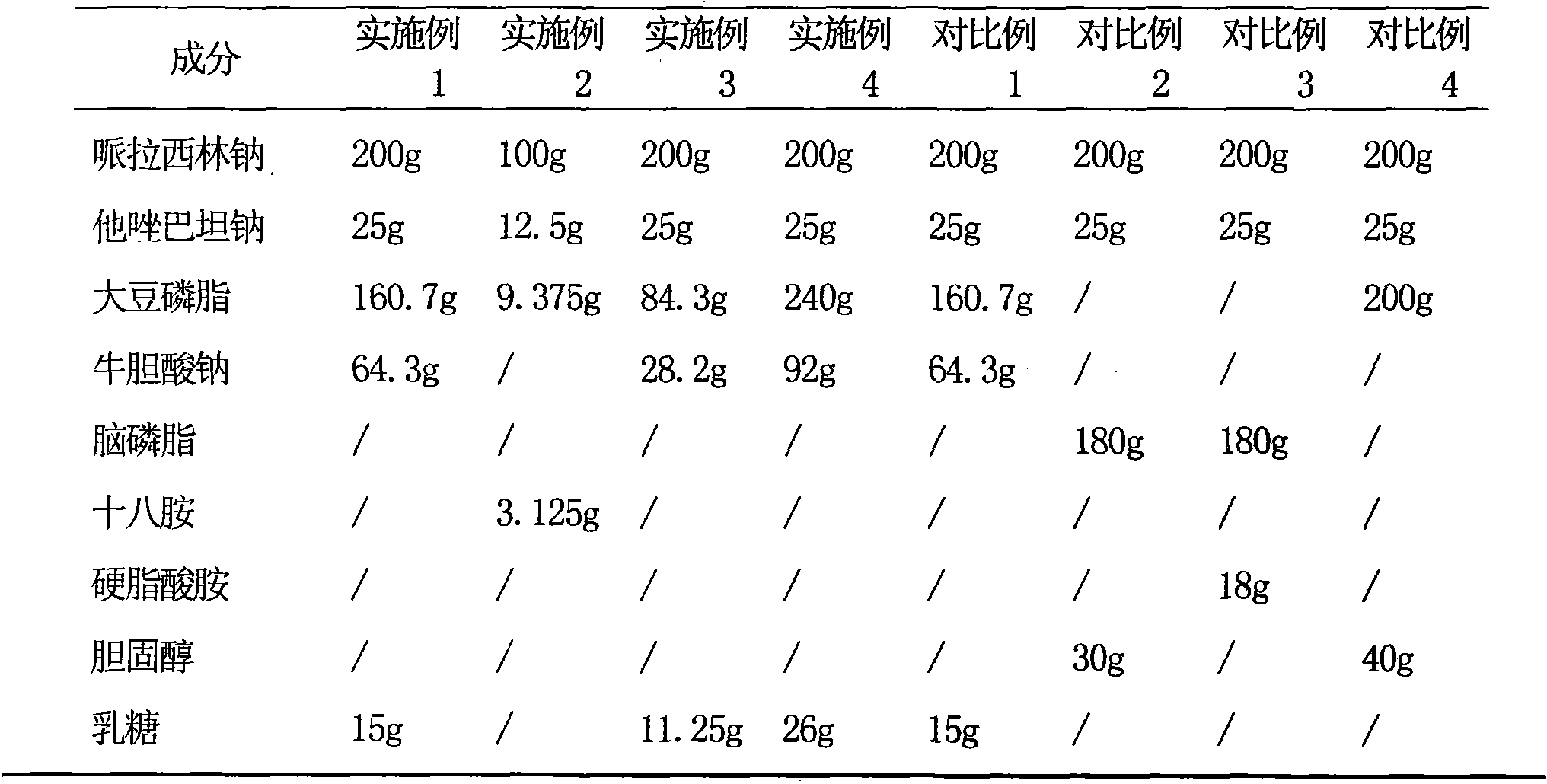
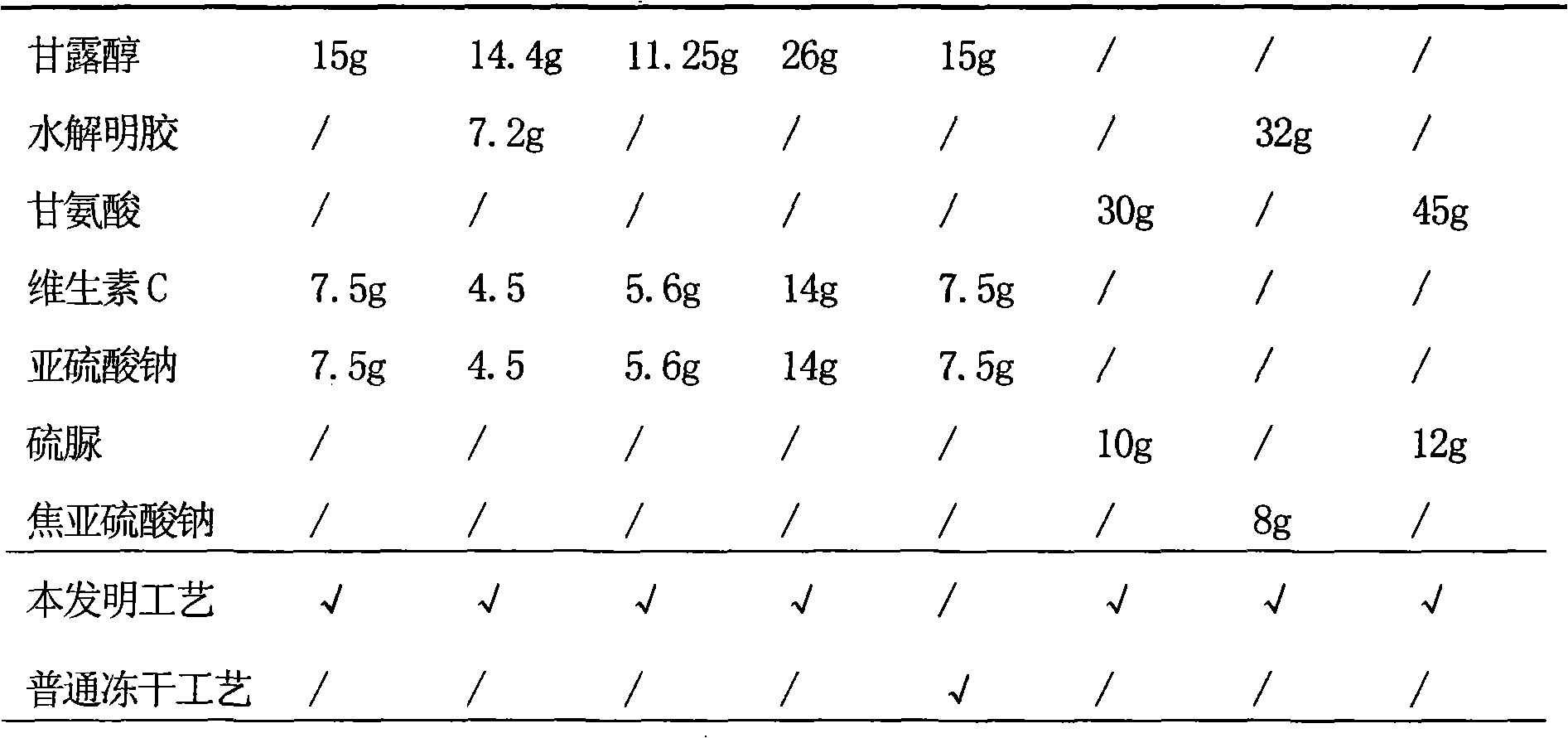
[0032] Prescription (100 bottles)
[0033] Piperacillin Sodium 200g
[0034] Tazobactam Sodium 25g
[0035] Soy Lecithin 160.7g
[0036] Sodium Taurocholate 64.3g
[0037] Lactose 15g
[0038] Mannitol 15g
[0039] Sodium sulfite 7.5g
[0040] Vitamin C 7.5g
[0041] making process:
[0042] (1) 160.7g soybean lecithin, 64.3g sodium taurocholate are dissolved in 500ml ethanol solution, then heated and evaporated to get rid of organic solvent completely, make blank lipid film;
[0043] (2) Add 300ml of phosphate buffer solution with a pH value of 6.5 to the blank lipid film and mix, place in a high-speed homogenizer to stir and emulsify evenly, then add 200g piperacillin sodium, 25g tazobactam sodium and 15g lactose , 15g of mannitol, 7.5g of sodium sulfite, and 7.5g of vitamin C were placed in a water bath at 70°C for 20 minutes of ultrasonic insulatio...
Embodiment 2
[0048] Example 2 Preparation of Piperacillin Sodium-Tazobactam Sodium Pharmaceutical Composition Liposomal Injection
[0049] Prescription (100 bottles)
[0050] Piperacillin Sodium 100g
[0051] Tazobactam Sodium 12.5g
[0052] Soy Lecithin 9.375g
[0053] Octadecylamine 3.125g
[0054] Hydrolyzed gelatin 7.2g
[0055] Mannitol 14.4g
[0056] Vitamin C 4.5g
[0057] Sodium sulfite 4.5g
[0058] making process:
[0059] (1) Dissolving 9.375g soybean lecithin and 3.125g octadecylamine in 200ml chloroform solution, then heating and evaporating to get rid of the organic solvent completely to obtain a blank lipid film;
[0060] (2) Add 300ml of phosphate buffer solution with a pH value of 7.5 to the blank lipid film and mix, place in a high-speed homogenizer to stir and emulsify evenly, then add 100g piperacillin sodium, 12.5g tazobactam sodium and 7.2 G hydrolyzed gelatin, 14.4g mannitol, 4.5g vitamin C, and 4.5g sodium sulfite were placed in a water bath at 70°C for 30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com