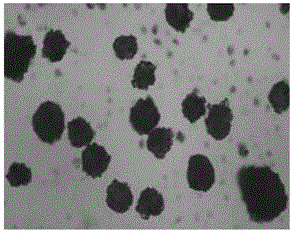
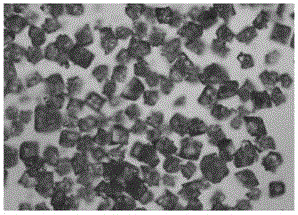
Piperacillin sodium tazobactam sodium preparation for injection and preparation method thereof
A technology of tazobactam sodium and piperacillin sodium, which is applied in the field of medicine to achieve low volatility, improve quality, and reduce agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) In the reaction flask, add 30 ml of dichloromethane, add 4.26 g of 2,3-dioxo-4-ethylpiperazine, and 2.52 g of sodium bicarbonate (dissolved in water), cool to -10°C, and stir to separate 2.97 g of solid phosgene was added three times, the temperature was raised to 40°C, the reaction was incubated for 1.5 hours, filtered, and the filtrate was used for later use;
[0030] (2) In the reaction flask, add 100 ml of dichloromethane, add 10.07 g of ampicillin trihydrate, dropwise add triethylamine to make the solution clear; add the filtrate of step (1), and react at 20°C to 25°C for 1 hour; Add hydrochloric acid dropwise, adjust the pH to 1.5-2.0, stand for stratification, discard the water layer; add 200 ml of 2% sodium bicarbonate dropwise to the dichloromethane layer, let stand for stratification, add 10 g of activated carbon to the water layer, stir for 10 minutes, and then filter ; Take the filtrate, control the temperature to be 15-20°C, slowly add hydrochloric acid...
Embodiment 2
[0034] (1) In the reaction flask, add 30 ml of dichloromethane, add 4.26 g of 2,3-dioxo-4-ethylpiperazine, and 2.52 g of sodium bicarbonate (dissolved in water), cool to -10°C, and stir to separate 2.97 g of solid phosgene was added three times, the temperature was raised to 40°C, the reaction was incubated for 1.5 hours, filtered, and the filtrate was used for later use;
[0035] (2) In the reaction flask, add 100 ml of dichloromethane, add 10.07 g of ampicillin trihydrate, dropwise add triethylamine to make the solution clear; add the filtrate of step (1), and react at 20°C to 25°C for 1 hour; Add hydrochloric acid dropwise, adjust the pH to 1.5-2.0, stand for stratification, discard the water layer; add 200 ml of 2% sodium bicarbonate dropwise to the dichloromethane layer, let stand for stratification, add 10 g of activated carbon to the water layer, stir for 10 minutes, and then filter Take the filtrate, control the temperature to be 15~20℃, slowly add hydrochloric acid dr...
Embodiment 3
[0040] (1) In the reaction flask, add 30 ml of dichloromethane, add 4.26 g of 2,3-dioxo-4-ethylpiperazine, and 2.52 g of sodium bicarbonate (dissolved in water), cool to -10°C, and stir to separate 2.97 g of solid phosgene was added three times, the temperature was raised to 50 °C, the reaction was incubated for 1.5 hours, filtered, and the filtrate was used for later use;
[0041](2) In the reaction flask, add 100 ml of dichloromethane, add 12.07 g of ampicillin trihydrate, dropwise add triethylamine to make the solution clarified; add step (1) filtrate, and react at 20°C to 25°C for 1 hour; Add hydrochloric acid dropwise, adjust the pH to 1.5-2.0, stand for stratification, discard the water layer; add 200 ml of 2% sodium bicarbonate dropwise to the dichloromethane layer, let stand for stratification, add 10 g of activated carbon to the water layer, stir for 10 minutes, and then filter Take the filtrate, control the temperature to be 15~20℃, slowly add hydrochloric acid dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com