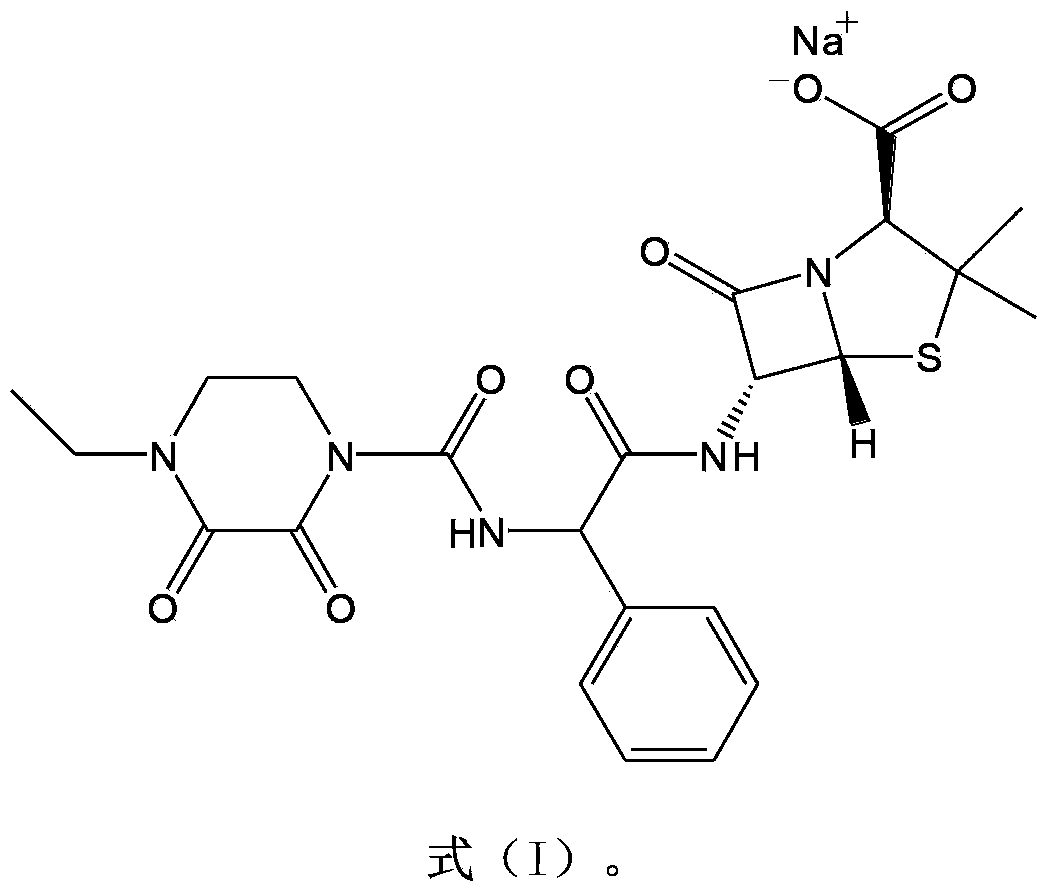
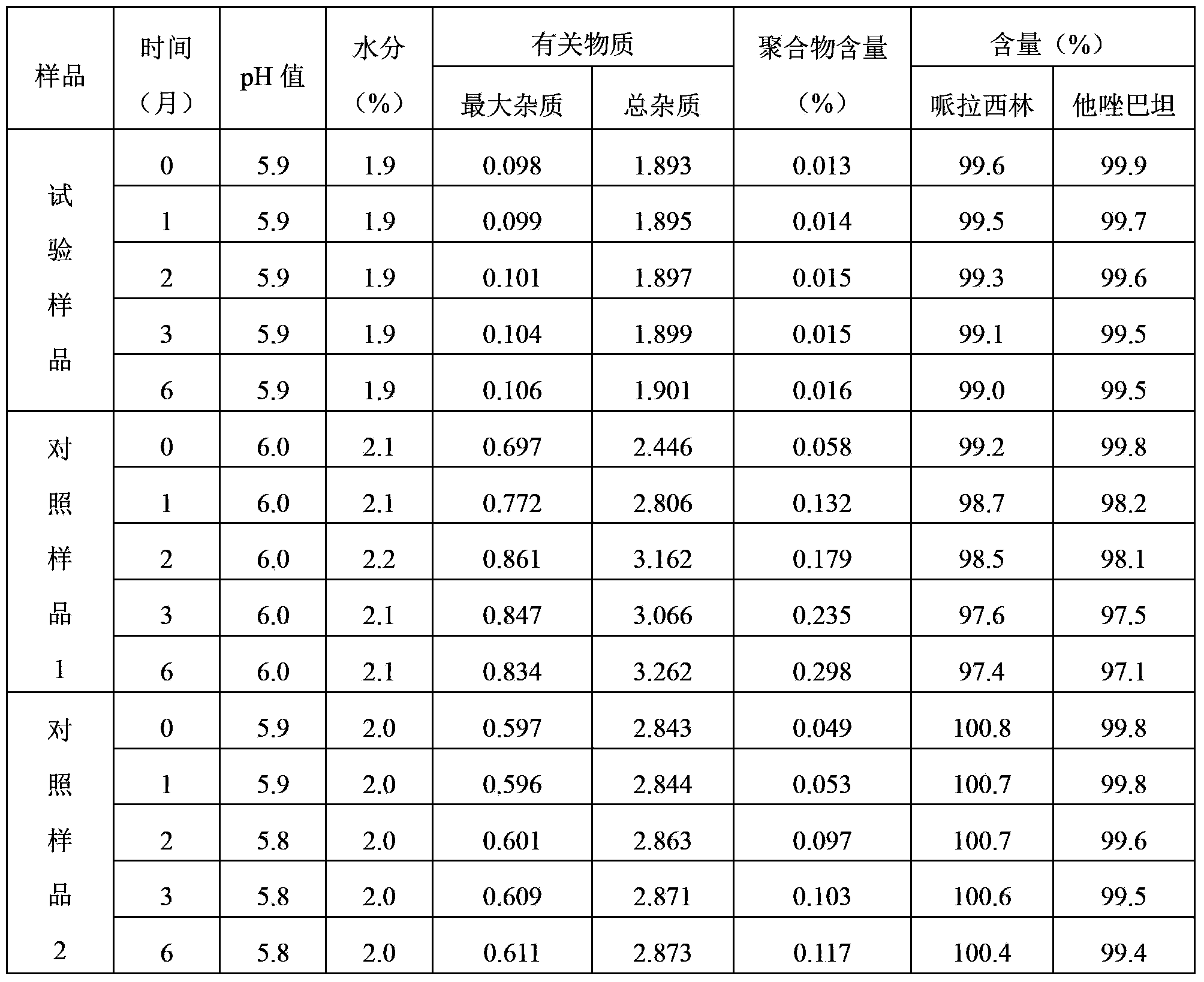
Piperacillin sodium-tazobactam sodium medicine composition and preparation method thereof
A technology of piperacillin sodium and tazobactam sodium, applied in the field of medicine, can solve the problems of large harm to patients, poor stability of preparations, rash or anaphylactic shock, etc., and achieve the effect of low polymer content and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1, piperacillin sodium
[0042] 1) Prepare a mixed solvent with isopropanol and N-methylpyrrolidone at a volume ratio of 3:1;
[0043] 2) Take 100 g of the piperacillin sodium raw material, dissolve it in 1000 ml of the mixed solvent of isopropanol and N-methylpyrrolidone in step 1), and stir until completely dissolved to obtain a piperacillin sodium solution;
[0044] 3) At a temperature of 3°C and a stirring speed of 2200r / min, add the piperacillin sodium solution in step 2) into acetone (the volume ratio of acetone to piperacillin sodium solution is 18:1), and mix to form suspension;
[0045]4) After the suspension was aged for 10 minutes, suction filtered, washed, and then the filter cake was vacuum-dried at 45°C to obtain a white crystalline powder, which was the piperacillin sodium.
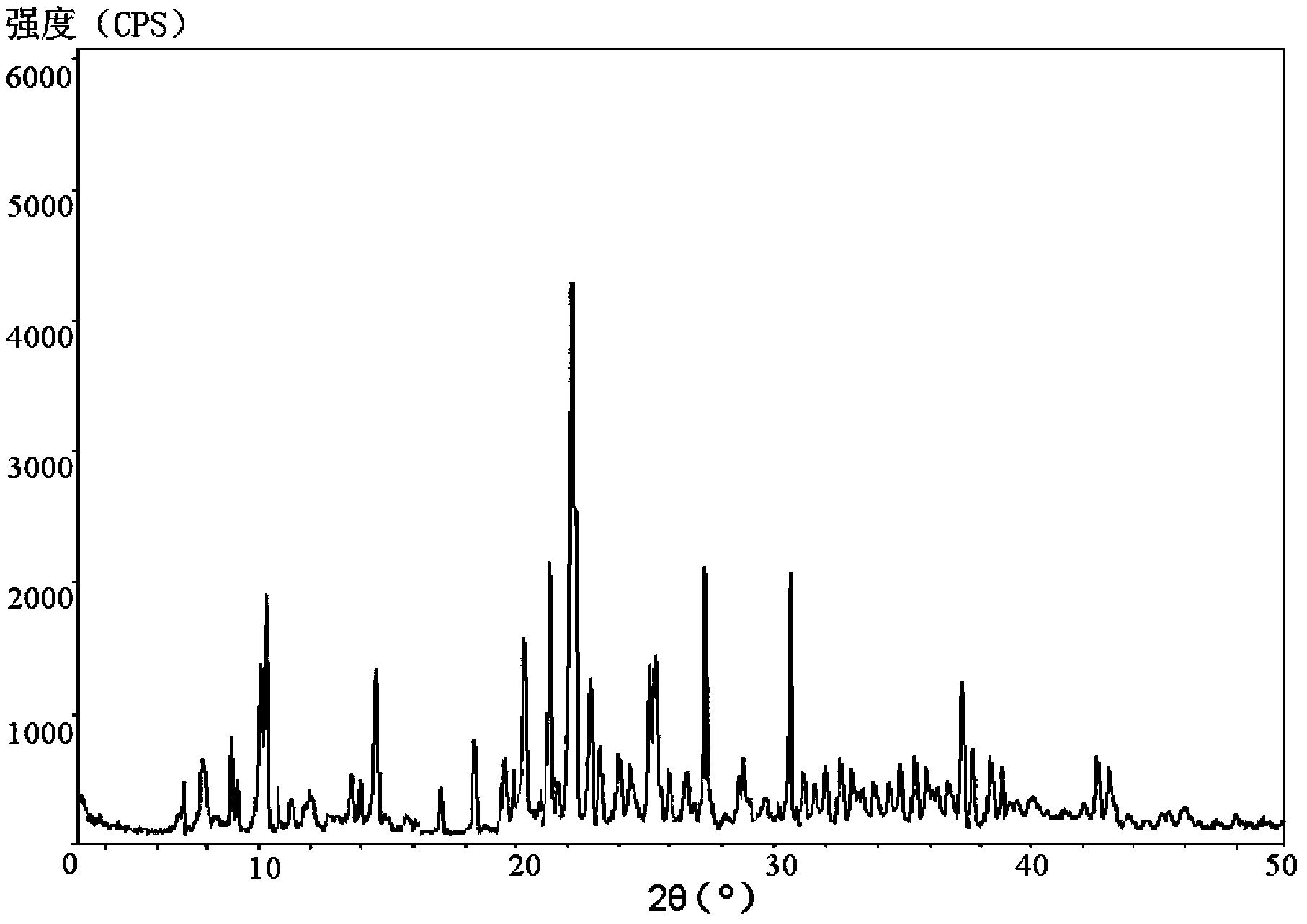
[0046] The X-ray powder diffraction pattern that the obtained piperacillin sodium is measured with powder X-ray diffractometry is as follows figure 1...
Embodiment 2
[0047] The preparation of embodiment 2, piperacillin sodium
[0048] 1) Mix isopropanol and N-methylpyrrolidone at a volume ratio of 5:1 to make a mixed solvent;
[0049] 2) Take 50 g of the piperacillin sodium raw material, dissolve it in 1000 ml of the mixed solvent of isopropanol and N-methylpyrrolidone in step 1), stir until completely dissolved, and obtain the piperacillin sodium solution;
[0050] 3) Add the piperacillin sodium solution in step 2) into acetone (the volume ratio of acetone to piperacillin sodium solution is 22:1) at a temperature of 8°C and a stirring speed of 1800r / min, and mix to form suspension;
[0051] 4) After the suspension was aged for 5 minutes, suction filtered, washed, and then the filter cake was vacuum-dried at 40°C to obtain a white crystalline powder, which was the piperacillin sodium.
[0052] The X-ray powder diffraction pattern that the obtained piperacillin sodium is measured with powder X-ray diffractometry is as follows figure 1 Sh...
Embodiment 3
[0053] The preparation of embodiment 3, piperacillin sodium
[0054] 1) Mix isopropanol and N-methylpyrrolidone at a volume ratio of 4:1 to make a mixed solvent;
[0055] 2) Take 80 g of the piperacillin sodium raw material, dissolve it in 1000 ml of the mixed solvent of isopropanol and N-methylpyrrolidone in step 1), and stir until completely dissolved to obtain the piperacillin sodium solution;
[0056] 3) Add the piperacillin sodium solution in step 2) into acetone (the volume ratio of acetone to piperacillin sodium solution is 20:1) at a temperature of 6°C and a stirring speed of 2000r / min, and mix to form suspension;
[0057] 4) After the suspension was aged for 8 minutes, suction filtered, washed, and then the filter cake was vacuum-dried at 42°C to obtain a white crystalline powder, which was the piperacillin sodium.
[0058] The X-ray powder diffraction pattern that the obtained piperacillin sodium is measured with powder X-ray diffractometry is as follows figure 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com