Preparation method of piperacillin sodium and tazobactam sodium freeze-drying agent for injection
A technology of tazobactam sodium and piperacillin sodium, which is applied in the direction of freeze-dried transportation, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc. Limitation, long production cycle increase and other problems, to shorten the liquid preparation time, optimize the freeze-drying process, reduce the effect of growth exceeding the limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
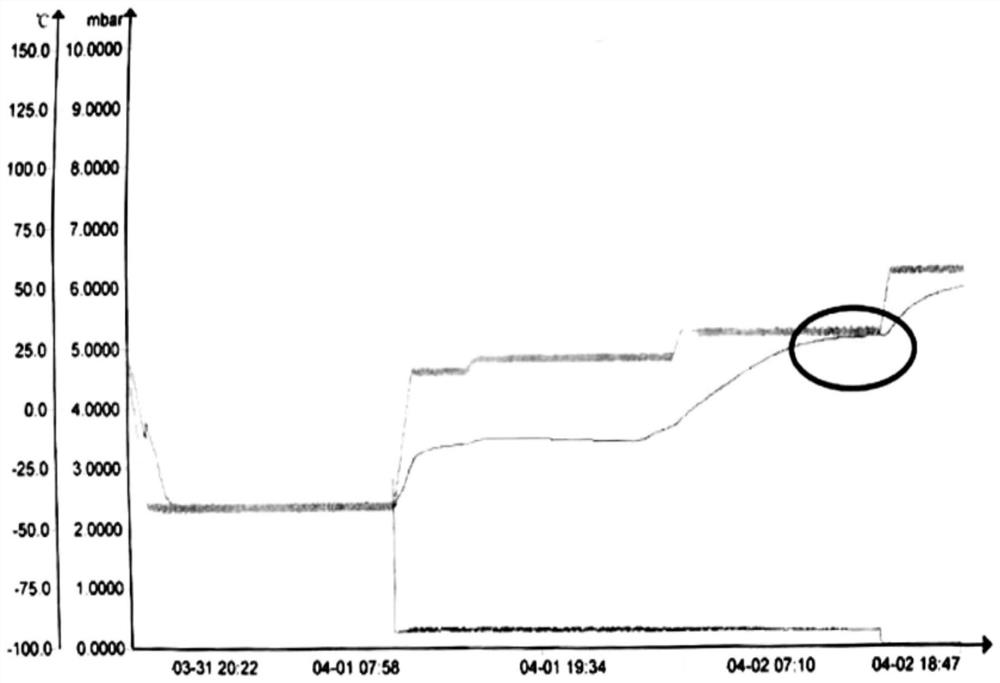
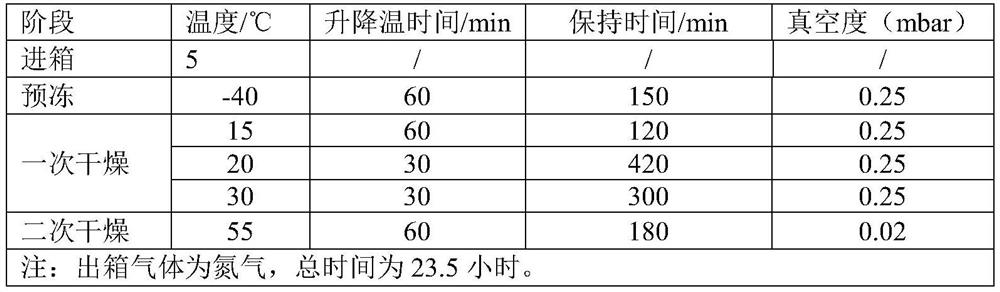
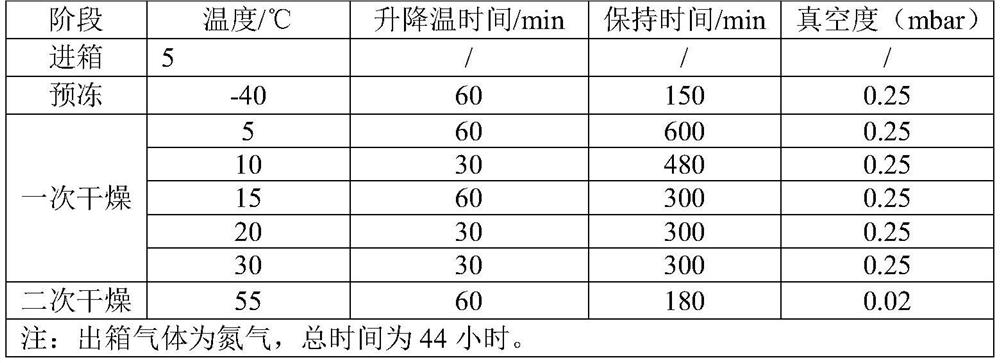
Image
Examples
preparation example Construction
[0029] The invention provides a preparation method for preparing a freeze-dried preparation of piperacillin sodium and tazobactam sodium for injection, wherein the mass ratio of the piperacillin sodium and tazobactam sodium is 8:1, specifically comprising the following steps:
[0030] 1. Weigh 55%-60% of the batch amount of ultra-pure water into the liquid mixing container, and cool down to 2-10°C. Weigh the mass of citric acid from the batch prescription, add it into the above-mentioned ultrapure water, stir until it dissolves completely, and the water temperature is within 2-10°C.
[0031] 2. Weigh about 16-17 parts of sodium bicarbonate powder into the above solution, stir until completely dissolved, stirring at a rate of 20Hz-40Hz, stirring for 15 minutes to make the pH 6.0-6.6; temperature within 2-10°C .
[0032] 3. Weigh the batch prescription amount of tazobactam and add it to the above solution several times, the actual addition time, and stir until it is completely ...
Embodiment 1
[0043] The preparation method of the piperacillin sodium-tazobactam sodium freeze-dried preparation for injection specifically comprises the following steps: 1. Weigh 55% of the batch amount of ultrapure water into a liquid preparation container, and cool down to 6°C. Weigh about 2.5 parts of citric acid in the batch recipe, add it into the above-mentioned high-purity water, and stir until it dissolves completely.
[0044] 2. Weigh about 16.5% sodium bicarbonate powder into the above solution and stir until completely dissolved. The stirring rate is 20Hz and the stirring time is 38min, so that the pH is 6.0-6.6; the pH is 6.30.
[0045] 3. Weigh the batch prescription amount of tazobactam and add it to the above solution several times, the addition time is 10 minutes, and stir at 20Hz until it is evenly mixed; the temperature of the solution is 3.4°C.
[0046] 4. Weigh the batch prescription amount of piperacillin and add it to the above solution several times, the addition ti...
Embodiment 2
[0058] The preparation method of the piperacillin sodium-tazobactam sodium freeze-dried preparation for injection specifically comprises the following steps: 1. Weigh 56% of the batch amount of ultrapure water into a liquid preparation container, and cool down to 8°C. Weigh about 2 to 3 parts of citric acid in batches, add to the above-mentioned high-purity water, and stir until completely dissolved.
[0059] 2. Weigh about 16 parts of sodium bicarbonate powder into the above solution, stir until completely dissolved, the stirring speed is 60rpm, and the stirring time is 10min, so that the pH is 6.0-6.6; the pH is 6.32.
[0060] 3. Weigh the amount of tazobactam in batches and add it to the above solution several times, the addition time is 8 minutes, and stir at 20Hz until it is evenly mixed; temperature: 5.2°C.
[0061] 4. Weigh the prescribed amount of piperacillin and add it to the above solution several times. The addition time is 18 minutes, and stir at 20Hz until it is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com